Sigma-1 receptor counteracts non-cell-autonomous poly-PR-induced astrocytic oxidative stress in C9orf72 ALS
- PMID: 40992079
- PMCID: PMC12495059
- DOI: 10.1016/j.redox.2025.103875
Sigma-1 receptor counteracts non-cell-autonomous poly-PR-induced astrocytic oxidative stress in C9orf72 ALS
Abstract
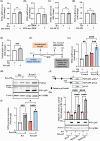
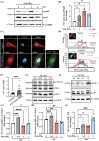
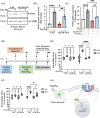
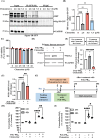
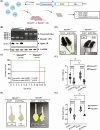
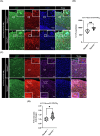
C9orf72-associated amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are characterized by the accumulation of toxic dipeptide repeat proteins (DPRs) generated from G4C2 hexanucleotide repeat expansions. Among these, the arginine-rich poly-PR (proline-arginine) species is the most neurotoxic, eliciting glial activation and neuroinflammation via non-cell-autonomous mechanisms. Although growing evidence implicates glial cells, particularly astrocytes, in disease progression, the molecular pathways linking neuron-derived poly-PR to astrocyte-mediated oxidative stress remain poorly understood. We demonstrate that exogenous poly-PR induces robust NOX4 expression and hydrogen peroxide (H2O2) production in astrocytes through activation of the IKK/IκB/NF-κB p65 signaling pathway. Mechanistically, poly-PR promotes nuclear translocation of p65 and enhances its binding to the NOX4 promoter, thereby amplifying astrocytic oxidative stress. Overexpression of the Sigma-1 receptor (Sigma-1R), an endoplasmic reticulum-resident chaperone, significantly attenuates poly-PR-induced NOX4 transcription and reactive oxygen species (ROS) production by interacting with p65 and blocking its nuclear translocation, independently of upstream p65 phosphorylation. Notably, clemastine, a clinically approved Sigma-1R agonist, suppresses astrocytic NOX4 expression by disrupting p65 binding to the NOX4 promoter. In a mouse model of C9orf72 ALS, Sigma-1R deficiency exacerbates poly-PR-induced neurodegeneration, astrogliosis, and NOX4 upregulation, whereas Sigma-1R sufficiency confers neuroprotection and anti-inflammatory effects. This study identifies Sigma-1R as a critical modulator of non-cell-autonomous poly-PR toxicity and establishes its activation as a potent suppressor of astrocyte-derived oxidative stress. Our findings uncover a previously unrecognized glial mechanism driving C9orf72 ALS pathogenesis and support Sigma-1R activation, via clemastine, as a promising therapeutic strategy to mitigate neuroinflammation and disease progression.
Keywords: Astrocyte; C9orf72 ALS; NOX4; Non-cell-autonomous poly-PR; Sigma-1R.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no competing interests.
Figures




References
-
- Zhang Y.J., Guo L., Gonzales P.K., Gendron T.F., Wu Y., Jansen-West K., O'Raw A.D., Pickles S.R., Prudencio M., Carlomagno Y., Gachechiladze M.A., Ludwig C., Tian R., Chew J., DeTure M., Lin W.L., Tong J., Daughrity L.M., Yue M., Song Y., Andersen J.W., Castanedes-Casey M., Kurti A., Datta A., Antognetti G., McCampbell A., Rademakers R., Oskarsson B., Dickson D.W., Kampmann M., Ward M.E., Fryer J.D., Link C.D., Shorter J., Petrucelli L. Heterochromatin anomalies and double-stranded RNA accumulation underlie C9orf72 poly(PR) toxicity. Science. 2019;363(6428) - PMC - PubMed
-
- Lin C.Y., Wu H.C., Fu R.H., Weng E.F., Hsieh W.C., Su T.P., Wu H.E., Wang S.M. Sigma-1R-Pom121 axis preserves nuclear transport and integrity in poly-PR-induced C9orf72 ALS. Neurobiol. Dis. 2025;212 - PubMed
-
- Xu L., Wang D., Zhao L., Yang Z., Liu X., Li X., Yuan T., Wang Y., Huang T., Bian N., He Y., Chen X., Tian B., Liu Z., Luo F., Si W., Gao G., Ji W., Niu Y., Wei J. C9orf72 poly(PR) aggregation in nucleus induces ALS/FTD-related neurodegeneration in cynomolgus monkeys. Neurobiol. Dis. 2023;184 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

