Early detection, clinicopathological subtyping, and prognosis prediction for endometrial cancer patients using fragmentomics liquid biopsy assay
- PMID: 40992272
- PMCID: PMC12495095
- DOI: 10.1016/j.esmoop.2025.105770
Early detection, clinicopathological subtyping, and prognosis prediction for endometrial cancer patients using fragmentomics liquid biopsy assay
Abstract
Background: Endometrial cancer (EC) is among the most prevalent gynecological malignancies worldwide. This study explores the use of cell-free DNA (cfDNA) fragmentomics to develop a non-invasive liquid biopsy assay, aiming to improve early detection, subtyping, and prognostication of EC, thereby enhancing therapeutic outcomes and reducing associated mortality.
Materials and methods: A cohort of 120 patients with diagnosed EC and 120 healthy volunteers was used to develop a novel non-invasive liquid biopsy assay for EC. Five distinct fragmentomic features were analyzed from preoperative plasma samples using low-pass whole-genome sequencing. Ensemble models were created by integrating base models that utilized four different machine learning algorithms for early cancer detection, clinicopathological subtyping, and prediction of recurrence-free survival. An independent test cohort of 62 EC patients and 62 healthy controls was used to assess the final ensemble model's performance.
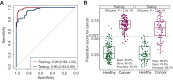
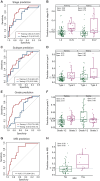
Results: The liquid biopsy assay demonstrated high efficacy in early EC detection, achieving an area under the curve (AUC) of 0.96, with 75.8% sensitivity and 96.8% specificity in the independent test cohort. Consistent sensitivities were observed across EC stages I-IV at 74.4%, 85.7%, 75%, and 75%, respectively. The assay moderately predicted clinicopathological features including stage (AUC = 0.72), histological subtypes (AUC = 0.73), and microsatellite instability status (AUC = 0.77). The model also effectively predicted recurrence-free survival, identifying high-risk patients [hazard ratio (HR) 8.6, P < 0.001]. Additionally, similarity network fusion stratified patients into high- and low-risk clusters, with high-risk individuals exhibiting a notably increased recurrence risk (HR 6.2, P = 0.049). Patients identified as high-risk by both methods exhibited an even greater risk (HR 10.1, P < 0.0001) for recurrence.
Conclusions: This DECIPHER-UCEC-2 study (Detecting Early Cancer by Inspecting ctDNA Features) demonstrates that by integrating cfDNA fragmentomics with machine learning, our liquid biopsy assay shows significant promise for EC's early detection, subtyping, and prognosis, potentially paving the way for enhanced patient outcomes.
Keywords: cell-free DNA; early diagnosis; endometrial cancer; fragmentomics; whole-genome sequencing.
Copyright © 2025 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures



References
-
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. - PubMed
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. - PubMed
-
- Oaknin A., Bosse T.J., Creutzberg C.L., et al. Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(9):860–877. - PubMed
-
- Frias-Gomez J., Benavente Y., Ponce J., et al. Sensitivity of cervico-vaginal cytology in endometrial carcinoma: a systematic review and meta-analysis. Cancer Cytopathol. 2020;128(11):792–802. - PubMed
-
- Jacobs I., Gentry-Maharaj A., Burnell M., et al. Sensitivity of transvaginal ultrasound screening for endometrial cancer in postmenopausal women: a case-control study within the UKCTOCS cohort. Lancet Oncol. 2011;12(1):38–48. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

