Mechanically activated snai1b coordinates the initiation of myocardial delamination for trabeculation
- PMID: 40993149
- PMCID: PMC12460811
- DOI: 10.1038/s41467-025-62285-w
Mechanically activated snai1b coordinates the initiation of myocardial delamination for trabeculation
Abstract
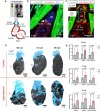
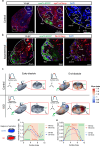
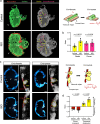
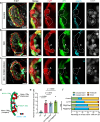
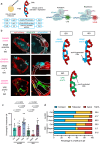
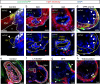
During development, myocardial contractile force and intracardiac hemodynamic shear stress coordinate the initiation of trabeculation. While Snail family genes are well-recognized transcription factors of epithelial-to-mesenchymal transition, snai1b-positive cardiomyocytes are sparsely distributed in the ventricle of zebrafish at 4 days post-fertilization. Isoproterenol treatment significantly increases the number of snai1b-positive cardiomyocytes, of which 80% are Notch-negative. CRISPR-activation of snai1b leads to 51.6% cardiomyocytes forming trabeculae, whereas CRISPR-repression reduces trabecular cardiomyocytes to 6.7% under isoproterenol. In addition, 36.7% of snai1b-repressed cardiomyocytes undergo apical delamination. 4-D strain analysis demonstrates that isoproterenol increases the myocardial strain along radial trabecular ridges in alignment with the snai1b expression and Notch-ErbB2-mediated trabeculation. Single-cell and spatial transcriptomics reveal that these snai1b-positive cardiomyocytes are devoid of some epithelial-to-mesenchymal transition-related phenotypes, such as Col1a2 production and induction by ErbB2 or TGF-β. Thus, we uncover snai1b-positive cardiomyocytes that are mechanically activated to initiate delamination for cardiac trabeculation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
- R01HL159970/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL159970/HL/NHLBI NIH HHS/United States
- R01 HL129727/HL/NHLBI NIH HHS/United States
- R01 HL165318/HL/NHLBI NIH HHS/United States
- T32HL144449/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

