Reprogramming neuroblastoma by diet-enhanced polyamine depletion
- PMID: 40993392
- PMCID: PMC12527938
- DOI: 10.1038/s41586-025-09564-0
Reprogramming neuroblastoma by diet-enhanced polyamine depletion
Abstract
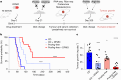
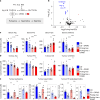
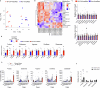
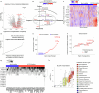
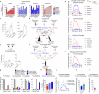
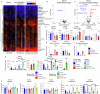
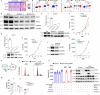
Neuroblastoma is a highly lethal childhood tumour derived from differentiation-arrested neural crest cells1,2. Like all cancers, its growth is fuelled by metabolites obtained from either circulation or local biosynthesis3,4. Neuroblastomas depend on local polyamine biosynthesis, and the inhibitor difluoromethylornithine has shown clinical activity5. Here we show that such inhibition can be augmented by dietary restriction of upstream amino acid substrates, leading to disruption of oncogenic protein translation, tumour differentiation and profound survival gains in the Th-MYCN mouse model. Specifically, an arginine- and proline-free diet decreases the amount of the polyamine precursor ornithine and enhances tumour polyamine depletion by difluoromethylornithine. This polyamine depletion causes ribosome stalling, unexpectedly specifically at codons with adenosine in the third position. Such codons are selectively enriched in cell cycle genes and low in neuronal differentiation genes. Thus, impaired translation of these codons, induced by combined dietary and pharmacological intervention, favours a pro-differentiation proteome. These results suggest that the genes of specific cellular programmes have evolved hallmark codon usage preferences that enable coherent translational rewiring in response to metabolic stresses, and that this process can be targeted to activate differentiation of paediatric cancers.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: J.D.R. is a member of the Rutgers Cancer Institute of New Jersey and the University of Pennsylvania Diabetes Research Center; a co-founder, director and stockholder in Raze Therapeutics and Farber Partners; a co-founder and stockholder in Fargo Biotechnologies; and an advisor and stockholder in Empress Therapeutics, Bantam Pharmaceuticals, Faeth Therapeutics, Colorado Research Partners and Rafael Pharmaceuticals. The University of Zürich has filed a provisional patent on combining difluormethylornithine with amino acid manipulations for therapeutic use.
Figures









Update of
-
Reprogramming neuroblastoma by diet-enhanced polyamine depletion.bioRxiv [Preprint]. 2024 Jan 8:2024.01.07.573662. doi: 10.1101/2024.01.07.573662. bioRxiv. 2024. Update in: Nature. 2025 Oct;646(8085):707-715. doi: 10.1038/s41586-025-09564-0. PMID: 38260457 Free PMC article. Updated. Preprint.
References
-
- Oesterheld, J. et al. Eflornithine as postimmunotherapy maintenance in high-risk neuroblastoma: externally controlled, propensity score-matched survival outcome comparisons. J. Clin. Oncol.42, 90–102 (2024).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

