Machine learning model to predict mortality in patients with skin and soft tissue infection in emergency department
- PMID: 40993724
- PMCID: PMC12462322
- DOI: 10.1186/s13049-025-01463-7
Machine learning model to predict mortality in patients with skin and soft tissue infection in emergency department
Abstract
Background: Accurately predicting mortality in patients with skin and soft-tissue infections (SSTIs) remains challenging. Machine learning models offer rapid processing, algorithmic impartiality, and strong predictive accuracy, which may improve early risk stratification in the emergency department (ED).
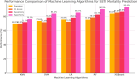
Methods: We retrospectively analyzed clinical data from 1,294 ED patients diagnosed with SSTIs between March 2015 and December 2020. Five machine learning algorithms-logistic regression (LR), k-nearest neighbours (KNN), support vector machine (SVM), random forest (RF), and Extreme Gradient Boosting (XGBoost)-were developed using 20 candidate variables, with model performance evaluated in independent runs. A simplified XGBoost model using only the six most influential predictors was also derived for bedside application.
Results: Among the five models, XGBoost achieved the highest performance (AUC = 0.892, sensitivity = 86.9%, specificity = 93.4%). The streamlined six-variable XGBoost model further improved predictive metrics (AUC = 0.922, sensitivity = 88.5%, specificity = 95.4%), matching or slightly surpassing the full model while reducing data requirements.
Conclusions: XGBoost outperformed LR, KNN, SVM, and RF in predicting SSTI mortality, offering both higher accuracy and operational efficiency. Its sequential tree-building, regularization, and robust handling of missing data enable superior discrimination in tabular clinical datasets. The simplified model, requiring only standard admission variables, provides a fast, cost-effective, and highly accurate tool for early identification of high-risk patients in the ED.
Keywords: Artificial intelligence; Machine learning; Mortality; Skin and soft tissue infection.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in accordance with the principles of the Declaration of Helsinki. Ethical approval was obtained from the Institutional Review Board of Chang Gung Memorial Hospital (No. 202302020B0). The IRB confirm that the data was anonymized or maintained with confidentiality. Consent to participate was not applicable and waived by Institutional review board of Chia-yi Chang Gung Memorial Hospital due to its a low risk retrospective medical chart review study. Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

