Reduced synaptic vesicle protein 2A in extracellular vesicles and brains of Alzheimer's disease: associations with Aβ, tau, synaptic proteins and APOE ε4
- PMID: 40993829
- PMCID: PMC12459057
- DOI: 10.1186/s40035-025-00508-2
Reduced synaptic vesicle protein 2A in extracellular vesicles and brains of Alzheimer's disease: associations with Aβ, tau, synaptic proteins and APOE ε4
Abstract
Background: Alzheimer's disease (AD) is characterized by accumulation of amyloid-β (Aβ) plaques, tau neurofibrillary Tangles and synaptic dysfunction. The aim of this study was to map the distributions of synaptic vesicle protein 2A (SV2A) and other synaptic proteins in the brain and the brain-derived extracellular vesicles (BDEVs) of AD patients, analyze their associations with Aβ, tau, and the apolipoprotein E (APOE) ε4 allele, and investigate the biological role of SV2A.
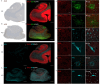
Methods: Mass spectrometry-based proteomics of BDEVs and immunohistochemistry staining were conducted on postmortem brain samples from 57 AD patients and 48 nondemented controls. The levels of SV2A, synaptophysin (SYP), and other synaptic proteins in the brain tissues and the BDEVs, and their associations with Aβ, tau (phospho-tau and Braak stages), other proteins and the APOE ε4 allele, were analyzed.
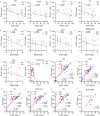
Results: SV2A levels were significantly lower in AD patients than in nondemented controls, particularly in the hippocampus and entorhinal cortex. APOE ε4 carriers presented further reductions in SV2A levels compared with noncarriers. The SV2A levels in BDEVs and brain tissues were positively correlated with SYP levels and negatively correlated with Aβ and phospho-tau levels. Reductions in SV2A were associated with decreased levels of other synaptic proteins, such as synaptotagmins, GAP43, and SNAP25. SV2A emerged as a central hub with interactions with proteins from subnetworks related to synaptic vesicle formation and fusion.
Conclusion: SV2A levels in brain tissues and BDEVs are reduced in AD patients, particularly in those carrying the APOE ε4 allele, and are correlated with Aβ and tau pathologies. SV2A may serve as a valuable biomarker for monitoring synaptic dysfunction and progression in AD.
Keywords: APOE ε4; Alzheimer’s disease; Amyloid-β; Extracellular vesicles; Proteomics; Synaptic vesicle protein 2A; Synaptophysin; Tau.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All the autopsied human brain tissue experiments were carried out in accordance with ethical permission obtained from the regional human ethics committee and the medical ethics committee of the VU Medical Center for NBB tissue. The consent for participation was obtained from the NBB, Lille Neurobank and CHUV. Consent for publication: Consent for publication was obtained from NBB, Lille Neurobank and CHUV. Competing interests: CH and RMN are employees and shareholders of Neurimmune AG. The authors declare that they have no conflicts of interest.
Figures




References
-
- Tzioras M, McGeachan RI, Durrant CS, Spires-Jones TL. Synaptic degeneration in Alzheimer disease. Nat Rev Neurol. 2023;19(1):19–38. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

