Cannabizetol, a Novel Cannabinoid: Chemical Synthesis, Anti-inflammatory Activity and Extraction from Cannabis sativa L
- PMID: 40994228
- PMCID: PMC12560071
- DOI: 10.1021/acs.jnatprod.5c00826
Cannabizetol, a Novel Cannabinoid: Chemical Synthesis, Anti-inflammatory Activity and Extraction from Cannabis sativa L
Abstract
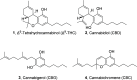
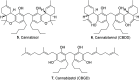
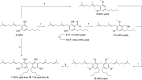
We report the first isolation of a previously unknown cannabinoid, cannabizetol (CBGD, 7), from Cannabis sativa extracts, representing the third member of the rare class of methylene-bridged dimeric cannabinoids. The availability of a chemically synthesized standard was crucial for its unequivocal identification, thus confirming the natural occurrence of this new compound. In addition to this structural discovery, we demonstrate that cannabizetol exhibits remarkable antioxidant and skin anti-inflammatory activity, significantly higher than that observed for the known dimeric cannabinoid cannabitwinol (CBDD, 6). These results highlight cannabizetol as a promising bioactive metabolite with potential dermatological applications. To further enable its study, we developed a continuous flow approach to optimize the preparation of these dimers, achieving a substantial reduction in reaction times.
Figures
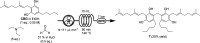
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

