Embedding a feruloyl esterase active site into a thermophilic endoxylanase scaffold for the degradation of feruloylated xylans
- PMID: 40994537
- PMCID: PMC12454871
- DOI: 10.1016/j.csbj.2025.09.003
Embedding a feruloyl esterase active site into a thermophilic endoxylanase scaffold for the degradation of feruloylated xylans
Abstract
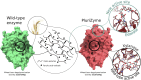
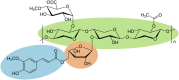
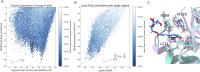
The structural complexity of xylan makes its complete degradation challenging. Strategies to improve its hydrolysis often requires enzyme cocktails with multiple specific activities or proteins harboring multiple catalytic domains. Here, we introduce a novel approach through the design of Xyn11m1, a multifunctional enzyme that combines endoxylanase and feruloyl esterase activities, two catalytic functions involved in the hydrolysis of feruloylated xylans. Using the PluriZyme concept, an artificial feruloyl esterase active site was engineered into the scaffold of a thermophilic glycoside hydrolase family 10 xylanase, Xyn11, from Pseudothermotoga thermarum. Computational design, guided by protein energy landscape exploration simulations, revealed a surface cavity that could accommodate feruloyl-L-arabinose and a xylopentaose (a 5-xylose xylan polymer) bearing a single feruloyl-L-arabinose substitution on the central xylose unit. This cavity was subsequently remodeled into a serine-histidine-aspartic/glutamic acid catalytic triad with feruloyl esterase activity. Molecular dynamics simulations confirmed the stability of the engineered active site. Xyn11m1 was successfully produced, crystallized, and characterized, and its xylanase activity at 90 °C against oat spelt xylan was comparable to that of the wild-type enzyme (713 ± 4 vs. 600 ± 8 units/mg), and it also displayed feruloyl esterase activity against methyl ferulate (140 ± 5 units/mg), a capability lacking in Xyn11. Notably, Xyn11m1 exhibited approximately 2.5-fold greater activity compared with Xyn11 (513 ± 27 vs. 222 ± 9 units/mg) against wheat bran xylan containing ferulic acid ester linked to arabinofuranosyl residues. This dual functionality enables efficient degradation of feruloylated xylans, highlighting the potential of PluriZymes to advance biomass deconstruction technologies.
Keywords: Feruloyl esterase; PluriZyme; Protein Engineering; Xylan; Xylanase.
© 2025 The Authors.
Conflict of interest statement
There are no known conflicts of interest associated with this publication.
Figures


References
LinkOut - more resources
Full Text Sources
