Impact of PARP Inhibitors on Health-related Quality of Life in Patients with Metastatic Castration-resistant Prostate Cancer: A Systematic Review and Meta-analysis
- PMID: 40994543
- PMCID: PMC12454867
- DOI: 10.1016/j.euros.2025.08.008
Impact of PARP Inhibitors on Health-related Quality of Life in Patients with Metastatic Castration-resistant Prostate Cancer: A Systematic Review and Meta-analysis
Abstract
Background and objective: PARP inhibitor (PARPi) agents, alone or in combination with antiandrogens, are considered a standard of care for patients with metastatic castration-resistant prostate cancer (mCRPC). This meta-analysis evaluates the impact of PARPi agents on health-related quality of life (HRQoL) using data from prospective trials.
Methods: In this prospectively registered systematic review and meta-analysis (PROSPERO: CRD420251011282), we searched the MEDLINE, Embase, and Web of Science databases in March 2025 for prospective trials assessing the impact of PARPi on patient HRQoL in mCRPC. Results for the mean differences (MD) in Functional Assessment of Cancer Therapy-Prostate (FACT-P) total scores at early and late time points were pooled using a random-effects model.
Key findings and limitations: Reports for seven prospective trials (2861 patients) were included. The PROfound trial (n = 387) demonstrated a clinically meaningful improvement in global HRQoL (MD from baseline: 6.7, 95% confidence interval [CI] 1.5-12), while the remaining six studies (n = 2474) did not show a clinically meaningful impact individually. In pooled analysis for the PROpel, MAGNITUDE, and NCT01972217 randomised controlled trials (n = 911), no clinically meaningful differences in the change in FACT-P total score were observed at early (weeks 8-9: MD -1.4, 95%CI -3.0 to 0.2) or late (weeks 96-101: MD -2.1, 95%CI -8.9 to 4.8) time points. Six studies were rated as having some/moderate concerns regarding bias, and one was considered at high risk of bias because of baseline stratification by symptom status.
Conclusions and clinical implications: We observed no statistically or clinically meaningful deterioration in HRQoL with PARPi treatment in patients with mCRPC, with one study suggesting a potential benefit. Predefined thresholds may not capture the individual and complex nature of HRQoL in the palliative mCRPC setting, where even modest changes can have important clinical and personal significance, which underscores the importance of nuanced interpretation of HRQoL data in guiding treatment decisions.
Patient summary: We did not find evidence that drugs called PARP inhibitors have a negative effect on health-related quality of life (HRQoL) for men with advanced prostate cancer. However, the current tools used to measure HRQoL may not fully reflect the individual and complex experiences of patients receiving palliative treatment for their cancer.
Keywords: EQ-5D-5L questionnaire; Functional Assessment of Cancer Therapy-Prostate; Health-related quality of life; Metastatic cancer; Prostate cancer.
© 2025 The Author(s).
Figures

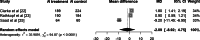
References
-
- Armstrong A.J., Lin P., Tombal B., et al. Five-year survival prediction and safety outcomes with enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer from the PREVAIL trial. Eur Urol. 2020;78:347–357. - PubMed
-
- Ryan C.J., Smith M.R., Fizazi K., et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–160. - PubMed
-
- de Bono J., Mateo J., Fizazi K., et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–2102. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

