Partial waiver of consent to overcome translational science barriers in neonatal clinical research
- PMID: 40994669
- PMCID: PMC12454670
- DOI: 10.1017/cts.2025.10122
Partial waiver of consent to overcome translational science barriers in neonatal clinical research
Abstract
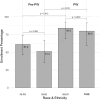
Prospective consent in neonatal research poses significant challenges, particularly during urgent, time-sensitive clinical windows of study enrollment. This is especially true at referral centers for large geographic regions. A partial waiver of consent offers a potential translational science approach to enhance access to research participation in critically ill neonates. We compared enrollment rates in a study evaluating pulse oximetry accuracy across neonates with varying skin pigmentation before and after implementing a partial waiver of consent. Overall enrollment increased significantly without creating a racial disparity in enrollment, thereby improving generalizability and efficiency in neonatal clinical research.
Keywords: Neonates; clinical trials; consent; enrollment; equity.
© The Author(s) 2025.
Conflict of interest statement
We have no conflicts of interest to disclose.
Figures
References
-
- HHS.gov Informed consent Office for Human Research Protections. 2025. (https://www.hhs.gov/ohrp/regulations-and-policy/guidance/faq/informed-co...) Accessed March 17, 2025.
LinkOut - more resources
Full Text Sources
