Machine Learning-Guided Identification of PET Hydrolases from Natural Diversity
- PMID: 40995134
- PMCID: PMC12455559
- DOI: 10.1021/acscatal.5c03460
Machine Learning-Guided Identification of PET Hydrolases from Natural Diversity
Abstract
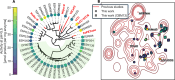
The enzymatic depolymerization of poly-(ethylene terephthalate) (PET) is emerging as a leading chemical recycling technology for waste polyester. As part of this endeavor, new candidate enzymes identified from natural diversity can serve as useful starting points for enzyme evolution and engineering. In this study, we improved upon HMM searches by applying an iterative machine learning strategy to identify 400 putative PET-degrading enzymes (PET hydrolases) from naturally occurring homologs. Using high-throughput (HTP) experimental techniques, we successfully expressed and purified >200 enzyme candidates and assayed them for PET hydrolysis activity as a function of pH, temperature, and substrate crystallinity. From this library, we discovered 91 previously unknown PET hydrolases, 35 of which retain activity at pH 4.5 on crystalline material, which are conditions relevant to developing more efficient commercial processes. Notably, four enzymes showed equal to or higher activity than LCC-ICCG, a benchmark PET hydrolase, at this challenging condition in our screening assay, and 11 of which have pH optima <7. Using these data, we identified regions of PETases statistically correlated to activity at lower pH. We additionally investigated the effect of condition-specific activity data on trained machine learning predictors and found a precision (putative hit rate) improvement of up to 30% compared to a Hidden Markov Model alone. Our findings show that by pointing enzyme discovery toward conditions of interest with multiple rounds of experimental and machine learning, we can discover large sets of active enzymes and explore factors associated with activity at those conditions.
Keywords: PET hydrolase; biocatalysis; high-throughput assay; interfacial biocatalysis; machine learning.
© 2025 The Authors. Published by American Chemical Society.
Figures





References
-
- Müller R., Schrader H., Profe J., Dresler K., Deckwer W.. Enzymatic Degradation of Poly(Ethylene Terephthalate):0 1Rapid Hydrolyse Using a Hydrolase from T. Fusca . Macromol. Rapid Commun. 2005;26(17):1400–1405. doi: 10.1002/marc.200500410. - DOI
-
- Sulaiman S., Yamato S., Kanaya E., Kim J.-J., Koga Y., Takano K., Kanaya S.. Isolation of a Novel Cutinase Homolog with Polyethylene Terephthalate-Degrading Activity from Leaf-Branch Compost by Using a Metagenomic Approach. Appl. Environ. Microbiol. 2012;78(5):1556–1562. doi: 10.1128/AEM.06725-11. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
