Photodissociation Dynamics in (N2)n+ Clusters
- PMID: 40995779
- PMCID: PMC12516711
- DOI: 10.1021/acs.jpca.5c05798
Photodissociation Dynamics in (N2)n+ Clusters
Abstract
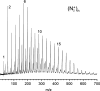
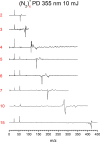
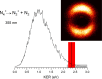
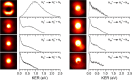
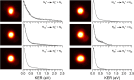
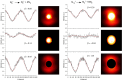
(N2)n+ cluster ions are produced and cooled in a pulsed-discharge supersonic expansion and studied with UV laser photodissociation and velocity-map imaging (VMI). All cluster sizes up to n = 15 absorb strongly near 355 nm, and those with n > 3 dissociate to produce both N2+ and N4+ photofragments. This suggests that the N4+ ion is the chromophore in the larger clusters, consistent with the previous optical spectroscopy and bond energy determinations. Photofragment imaging of N4+ produces an anisotropic distribution peaked along the laser polarization. Analysis of the maximum kinetic energy release produces a dissociation energy consistent with values determined in previous experiments. Dissociation of larger clusters produces N2+ with significant kinetic energy values that do not change appreciably with cluster size. This suggests that the N4+ core ion is not enclosed by the clustering of additional N2 molecules. N4+ fragments from larger clusters have somewhat lower kinetic energies than the N2+ fragments, consistent with recombination or partial caging after dissociative recoil. However, the kinetic energy release of N4+ is also considerable and it persists in the dissociation of larger clusters. This suggests that the N4+ ion in these clusters resides near the surface and that the photodissociation and recombination are mediated by this surface rather than by a true caging effect.
Figures
References
-
- Aplin K. L.. Composition and Measurement of Charged Atmospheric Clusters. Space Sci. Rev. 2008;137:213–224. doi: 10.1007/s11214-008-9397-1. - DOI
-
- Anicich V. G., Milligan D. B., Fairley D. A., McEwan M. J.. Termolecular Ion–Molecule Reactions in Titan’s Atmosphere, I. Icarus. 2000;146:118–124. doi: 10.1006/icar.2000.6353. - DOI
-
- Payzant J. D., Kebarle P.. Clustering Equilibrium N2 + + 2N2 = N4 + + N2 and the Bond Dissociation Energy of N4 + . J. Chem. Phys. 1970;53:4723–4724. doi: 10.1063/1.1674010. - DOI
-
- Smith D., Adams N. G., Miller T. M.. A Laboratory Study of the Reactions of N+, N2 +, N3 +, N4 +, O+, O2 + and NO+ Ions with Several Molecules at 300 K. J. Chem. Phys. 1978;69:308–318. doi: 10.1063/1.436354. - DOI
LinkOut - more resources
Full Text Sources

