Tryptophan catabolites from microbiota ameliorate immune-mediated hepatitis through activating aryl hydrocarbon receptor of T cells
- PMID: 40995824
- PMCID: PMC12477874
- DOI: 10.1080/19490976.2025.2557979
Tryptophan catabolites from microbiota ameliorate immune-mediated hepatitis through activating aryl hydrocarbon receptor of T cells
Abstract
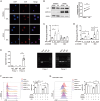
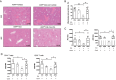
Intestinal dysbiosis and T cell-mediated immune attack are implicated in the pathogenesis of autoimmune hepatitis (AIH). However, the mechanisms by which microbiota-derived metabolites modulate immune homeostasis in AIH remain elusive. Here, we demonstrated that microbiota-derived indole-3-carboxaldehyde (ICA) was significantly reduced in patients with AIH. Treatment with ICA restricted the activation of effector T cells by activating AhR in T lymphocytes. Nuclear translocation of AhR induced the transcription of PI3K interacting protein 1 (Pik3ip1), which inhibited the PI3K/Akt/mTOR signaling pathway. In vivo supplementation of ICA suppressed effector T cells and mitigated the tissue damage and hepatic inflammation in two mouse models of T cell-mediated hepatitis. Importantly, T cell-specific deletion of AhR abrogated the protective effects of ICA in AIH-like mouse model. Finally, administration of Lactobacillus reuteri resulted in elevated level of ICA and protected mice from liver damage. Our data suggest that ICA supplementation ameliorates immune-mediated hepatitis through agonizing AhR in T cells, presenting a promising therapeutic strategy for AIH.
Keywords: Indole-3-carboxaldehyde; PI3K interacting protein 1; aryl hydrocarbon receptor; immune-mediated hepatitis; lactobacillus reuteri.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
