IL-21-reprogrammed Vδ1 T cells exert killing against solid tumors which is enhanced by CAR arming for off-the-shelf immunotherapy
- PMID: 40996786
- PMCID: PMC12477882
- DOI: 10.1080/2162402X.2025.2562210
IL-21-reprogrammed Vδ1 T cells exert killing against solid tumors which is enhanced by CAR arming for off-the-shelf immunotherapy
Abstract
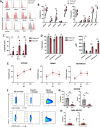
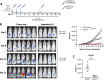
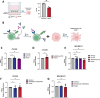
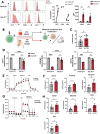
Cancer cell therapies have primarily focused on engineering autologous αβ T cells with chimeric antigen receptors (CARs), achieving clinical success against hematologic malignancies. However, their effectiveness against solid tumors is limited by challenges such as antigen escape, suppression by the metabolically hostile tumor microenvironment (TME), and manufacturing difficulties. γδ T cells are unconventional T cells with innate tumor-targeting capabilities independent of MHC class I, making them an emerging candidate for allogeneic cell therapy. While the Vδ1 T cell subset has shown promising anti-tumor killing their clinical application has been hindered by difficulties in achieving robust expansion for therapeutic use. Here, we evaluated the potential of K562 feeder cells expressing membrane-bound IL-21 (K562-mb-IL-21) to expand and activate γδ T cells from peripheral blood. Our findings show that this method preferentially expands Vδ1 T cells, resulting in an activated phenotype characterized by enhanced expression of NK cell activation receptors, innate cytotoxicity against breast and ovarian cancer cells, and sustained metabolic function in patient-derived ascites TME. When engineered with a CAR, Vδ1 T cells exhibited further enhanced anti-tumor efficacy in an immunodeficient NRG xenograft model of human ovarian cancer. These findings highlight K562-mb-IL-21 expanded peripheral blood Vδ1 T cells as a promising 'off-the-shelf' allogeneic therapy for solid tumors.
Keywords: CAR-T cells; Cell therapy; IL-21; metabolic fitness; solid tumors; γδ T cells.
Conflict of interest statement
MNK is a co-inventor of gamma delta T cell gene editing technology, he is also the co-founder and CSO of CARTx Therapeutics and owns stock in the company. All other authors declare no competing interests.
Figures

References
-
- Deusch K, Lüling F, Reich K, Classen M, Wagner H, Pfeffer K. A major fraction of human intraepithelial lymphocytes simultaneously expresses the γ/δ T cell receptor, the CD8 accessory molecule and preferentially uses the Vδ1 gene segment. Eur J Immunol. 1991;21(4):1053–1059. doi: 10.1002/eji.1830210429. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
