Pediatric Influenza-Associated Encephalopathy and Acute Necrotizing Encephalopathy - United States, 2024-25 Influenza Season
- PMID: 40996921
- PMCID: PMC12463191
- DOI: 10.15585/mmwr.mm7436a1
Pediatric Influenza-Associated Encephalopathy and Acute Necrotizing Encephalopathy - United States, 2024-25 Influenza Season
Abstract
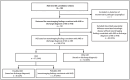
In January 2025, CDC received several reports of deaths among children aged <18 years with a severe form of influenza-associated encephalopathy (IAE) termed acute necrotizing encephalopathy (ANE). Because no national surveillance for IAE currently exists, CDC requested notification of U.S. pediatric IAE cases from clinicians and health departments during the 2024-25 influenza season, a high-severity season with a record number of pediatric influenza-associated deaths. Among 192 reports of suspected IAE submitted to CDC, 109 (57%) were categorized as IAE, 37 (34%) of which were subcategorized as ANE, and 72 (66%) as other IAE; 82 reports did not meet IAE criteria and were categorized as other influenza-associated neurologic disease. The median age of children with IAE was 5 years and 55% were previously healthy, 74% were admitted to an intensive care unit, and 19% died; 41% of children with ANE died. Only 16% of children with IAE who were vaccination-eligible had received the 2024-25 influenza vaccine. Health care providers should consider IAE in children with encephalopathy or altered level of consciousness and a recent or current febrile illness when influenza viruses are circulating. Annual influenza vaccination is recommended for all children aged ≥6 months to prevent influenza and associated complications, potentially including severe neurologic disease such as IAE and ANE.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. James W. Antoon reports institutional support from the National Institutes of Health (NIH) and participated on the AstraZeneca Advisory Board. Annabelle De St. Maurice reports receipt of honoraria from Merck Manuals. Ashleigh Faulstich reports grant support from the Nevada Office of State Epidemiology. Sue Hong reports uncompensated service as secretary/treasurer of the Pediatric Neurocritical Care Research Support Group. David A. Hunstad reports grant support from NIH, BioAge Laboratories, and Pfizer; honoraria from the University of Iowa; support for attending the International Pediatric Research Foundation; and leadership and stock options in BioVersys AG. Curi Kim reports support for attending the 2025 Council of State and Territorial Epidemiologists’ (CSTE) Conference. Scott Lindquist reports support for attending the 2025 CSTE Conference. Molly Wilson-Murphy reports financial support from the Boston Children’s Hospital Department of Neurology; honoraria from the American Academy of Neurology; support for meeting attendance through the Boston Children’s Hospital Trust; leadership in the American Academy of Neurology Neuroinfectious Disease Section Education Committee and the Child Neurology Society Neuroinfectious Disease Special Interest Group; and membership in the Acute Flaccid Myelitis Working Group. Samantha M. Olson reports travel and meeting support from the Gates Foundation. Adrienne G. Randolph reports institutional support from the National Institute of Allergy and Infectious Diseases and NIH; royalties from UpToDate Inc.; consulting fees from Thermo Fisher, Inc. and Inotrem Inc; participation in the REMAP-CAP trial advisory board; and leadership in the International Sepsis Forum. Suchitra Rao reports grant support from the National Heart, Lung, and Blood Institute, NIH, the Patient-Centered Outcomes Research Institute, and the Agency for Healthcare Research and Quality. Keith P. Van Haren reports grant support from NIH, IONIS Pharmaceuticals, and Minoryx; support to travel to Adrenoleukodystrophy (ALD) Connect; participation on a data and safety monitoring committee for a clinical trial sponsored by Calico Life Sciences and AbbVie, and unpaid leadership on the Board of Directors for ALD Connect and the Medical Science Advisory Board for the United Leukodystrophy Foundation. Andy Weigel reports support for attending the 2025 CSTE Conference. No other potential conflicts of interest were disclosed.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical