Efficacy and Safety of Nivolumab Monotherapy in Patients with High PD-1-Positive CD8/Treg Ratio in Advanced NSCLC and Gastric Cancer: A Phase II, Multicenter Study
- PMID: 40997059
- PMCID: PMC12525050
- DOI: 10.1158/2767-9764.CRC-25-0169
Efficacy and Safety of Nivolumab Monotherapy in Patients with High PD-1-Positive CD8/Treg Ratio in Advanced NSCLC and Gastric Cancer: A Phase II, Multicenter Study
Abstract
Purpose: It is challenging to identify the appropriate patients who benefit from anti-PD-1/PD-L1 monotherapy. For predicting effectiveness of anti-PD-1/PD-L1 monotherapy, this open-label phase II study (ONO-4538-88) evaluated the potential of the tumor-infiltrating lymphocyte (TIL) biomarker: the balance between cytotoxic T cells and regulatory T cells.
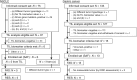
Patients and methods: Patients with advanced non-small cell lung cancer (NSCLC) or gastric cancer were screened between March 2021 and January 2022. Eligible patients who met the prespecified TIL biomarker criteria received nivolumab monotherapy. The primary endpoint was objective response rate (ORR). The secondary endpoints included overall survival and progression-free survival. Conventional biomarkers (tumor proportion score, combined positive score, tumor mutation burden, and microsatellite instability) were exploratorily analyzed, and safety was also assessed.
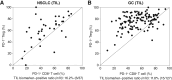
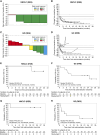
Results: Thirty-seven patients with NSCLC and 127 patients with gastric cancer were eligible for TIL analysis: 6 (16.2%) and 15 patients (11.8%) met the TIL biomarker criteria, respectively; a part of them were assessed. For NSCLC and gastric cancer, the ORR was 80% (4/5 patients) and 36.4% (4/11 patients), respectively; all the five patients and 5/11 patients had a reduction in tumor size, respectively; the median overall survival was not reached and 25 months, respectively; and the median progression-free survival was not reached and 5.59 months, respectively. Treatment-related adverse events occurred in 13/19 patients overall: 5/6 patients for NSCLC and 8/13 patients for gastric cancer.
Conclusions: Although the low positive rate of the TIL biomarker limits interpretation, the promising ORRs suggest signs of the TIL biomarker's predictability for nivolumab monotherapy.
Significance: In this phase II study, we examined the predictive utility of the TIL biomarker for nivolumab monotherapy. Although the positivity of the TIL biomarker was limited, the promising efficacy and safety profile in the TIL biomarker-positive patients may suggest the potential utility of the TIL biomarker.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
K. Shitara reports grants and personal fees from Astellas Pharma, Ono Pharmaceutical, Daiichi Sankyo, MSD, Amgen, and AstraZeneca, grants from PPD-SNBL K.K, TORAY, Taiho Pharmaceutical, Chugai Pharmaceutical, Eisai, PRA Health Sciences, and Syneos Health, and personal fees from Bristol Myers Squibb, Takeda, Novartis, Boehringer Ingelheim, Guardant Health Japan, Janssen, Zymeworks Biopharmaceuticals Inc., ALX Oncology Inc., Bayer, GlaxoSmithKline K.K., HEALIOS K.K., Moderna Inc., Arcus Biosciences Inc., and Eli Lilly outside the submitted work. M. Tamiya reports personal fees from Ono Pharmaceutical, MSD, AstraZeneca, Bristol Myers Squibb, Chugai Pharmaceutical, Eli Lilly, and Pfizer outside the submitted work. K. Okishio reports personal fees from Bristol Myers Squibb K.K, AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Takeda Pharmaceutical Company, Taiho Pharmaceutical Co., Ltd., Eli Lilly Japan K.K outside the submitted work. N. Seki reports grants and personal fees from Ono Pharmaceutical during the conduct of the study, as well as grants and personal fees from Eli Lilly, Chugai Pharmaceutical, Taiho Pharmaceutical, Pfizer, Nippon Kayaku, Takeda Pharmaceutical, Boehringer Ingelheim, Daiichi Sankyo, grants from Eisai and Shionogi, and personal fees from AstraZeneca, MSD, Bristol Myers Squibb, Novartis, Kyowa Kirin, Amgen, Johnson & Johnson, and Merck Biopharma outside the submitted work. H. Hara reports grants from Ono Pharmaceutical during the conduct of the study and grants from AstraZeneca, Merck Biopharma, MSD, Ono Pharmaceutical, Taiho Pharmaceutical, Daiichi Sankyo, BeiGene, Astellas Pharma, Bayer, Amgen, Chugai Pharma, Janssen Oncology, ALX Oncology, Bristol Myers Squibb, Jazz Pharmaceuticals, Oncolys Biopharma, Henlius, AbbVie, and Innovent outside the submitted work. Y. Narita reports grants from Chugai, MSD, Amgen, ONO Pharmaceutical, Astellas, Sanofi, Taiho, Eisai, Daiichi Sankyo, Novartis, and Pfizer, personal fees from Yakult Honsha, Taiho, Eli Lilly, Daiichi Sankyo, Ono Pharmaceutical, and Bristol Myers Squibb, and other support from Daiichi Sankyo outside the submitted work. Y. Tamura reports personal fees from MSD (Merck & Co., Inc.), AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., and Nippon Boehringer Ingelheim Co., Ltd. and grants and personal fees from Ono Pharmaceutical outside the submitted work. A. Tsuji reports personal fees from Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan Co., Ltd., Merck Biopharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Bristol Myers Squibb Corporation during the conduct of the study. H. Tanaka reports grants from Ono Pharmaceutical during the conduct of the study, as well as grants and personal fees from Ono Pharmaceutical, AstraZeneca, Chugai Pharmaceutical, MSD, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, Merck, Takeda pharmaceutical, Taiho Pharmaceutical, Amgen, and Boehringer Ingelheim, personal fees from Eisai, Novartis, Pfizer, Celltrion, and Sun pharmaceutical, and grants from AbbVie outside the submitted work. K. Yamaguchi reports grants from Ono Pharmaceutical Co., Ltd. during the conduct of the study, as well as personal fees from Daiichi Sankyo Co., Ltd., Chugai Pharmaceutical Co., Ltd., Bristol Myers Squibb K.K., Taiho Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd. and grants and personal fees from Astellas outside the submitted work. H. Izumi reports grants from Ono Pharmaceutical Company and grants from Bristol Myers Squibb during the conduct of the study, as well as grants and personal fees from Amgen, Takeda, Daiichi Sankyo, and AstraZeneca, grants from AbbVie, and ArriVent BioPharma, and personal fees from Chugai and Eli Lilly outside the submitted work. Y. Ushida reports other support from Ono Pharmaceutical Co., Ltd. outside the submitted work. H. Suna reports other from Ono Pharmaceutical Co., Ltd. outside the submitted work. No disclosures were reported by the other authors.
Figures
References
-
- Kang Y-K, Boku N, Satoh T, Ryu M-H, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

