Targeting prostaglandin E2 receptor 2 in Schwann cells inhibits inflammatory pain but not inflammation
- PMID: 40998803
- PMCID: PMC12462433
- DOI: 10.1038/s41467-025-63782-8
Targeting prostaglandin E2 receptor 2 in Schwann cells inhibits inflammatory pain but not inflammation
Abstract
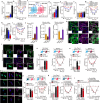
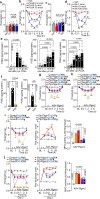
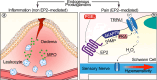
Analgesia by non-steroidal anti-inflammatory drugs (NSAIDs) is ascribed to inhibition of prostaglandin (PG) biosynthesis and ensuing inflammation. However, NSAIDs have life-threatening side effects, and inhibition of inflammation delays pain resolution. Decoupling the mechanisms underlying PG-evoked pain vs. protective inflammation would facilitate pain treatment. Herein, we reveal that selective silencing of the PGE2 receptor 2 (EP2) in Schwann cells via adeno-associated viral vectors abrogates the indomethacin-sensitive component of pain-like responses in mice elicited by inflammatory stimuli without affecting inflammation. In human Schwann cells and in mice, EP2 activation and optogenetic stimulation of adenylyl cyclase evokes a plasma membrane-compartmentalized cyclic adenosine monophosphate (cAMP) signal that, via A-kinase anchor protein-associated protein kinase A, sustains inflammatory pain-like responses, but does not delay their resolution. Thus, an unforeseen and druggable EP2 receptor in Schwann cells, via specific cAMP nanodomains, encodes PGE2-mediated persistent inflammatory pain but not PG-dependent protective inflammation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: N.W.B. is a founding scientist of Endosome Therapeutics and pHArm Therapeutics. P.G. is a member of the Scientific Advisory Board of Endosome Therapeutics. R.N., F.D.L. and P.G. are founding scientists of FloNext Srl. G.B. has been fully employed at FloNext Srl, Italy. The remaining authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

