Molecular basis of ParA ATPase activation by the CTPase ParB during bacterial chromosome segregation
- PMID: 40998818
- PMCID: PMC12462528
- DOI: 10.1038/s41467-025-63976-0
Molecular basis of ParA ATPase activation by the CTPase ParB during bacterial chromosome segregation
Abstract
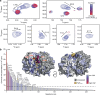
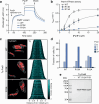
DNA segregation by bacterial ParABS systems is mediated by transient tethering interactions between nucleoid-bound dimers of the ATPase ParA and centromere (parS)-associated complexes of the clamp-forming CTPase ParB. The lifetime of these interactions is limited by the ParB-dependent activation of ParA ATPase activity. Here, we elucidate the functional interplay between ParA and ParB in the model bacterium Myxococcus xanthus. We demonstrate that the N-terminal ParA-binding motif of ParB associates with a conserved bipartite binding pocket at the ParA dimer interface, in a manner dependent on ParB clamp closure. Moreover, we show that ParB and non-specific DNA interact cooperatively with ParA and synergistically induce structural changes in its Walker A and Walker B motifs that correlate with the activation of ParA ATPase activity. These results advance our understanding of the mechanism underlying DNA transport by the ParABS system and may help to unravel the mode of action of related cargo-positioning systems.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Gerdes, K., Moller-Jensen, J. & Bugge Jensen, R. Plasmid and chromosome partitioning: surprises from phylogeny. Mol. Microbiol.37, 455–466 (2000). - PubMed
MeSH terms
Substances
Grants and funding
- 505997786 - GRK 2937/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 505997786 - GRK 2937/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 260989694/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 324652314/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 101097986 - C-SWITCH/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
LinkOut - more resources
Full Text Sources
Miscellaneous

