Use of a molecular syndromic panel for the etiological diagnosis of ventilator-associated bacterial pneumonia: impact on clinical outcomes and antibiotic use from a multicenter, prospective study
- PMID: 40999416
- PMCID: PMC12466008
- DOI: 10.1186/s13054-025-05632-z
Use of a molecular syndromic panel for the etiological diagnosis of ventilator-associated bacterial pneumonia: impact on clinical outcomes and antibiotic use from a multicenter, prospective study
Abstract
Background: Ventilator-associated bacterial pneumonia (VABP) is a common infection in critically ill patients in intensive care units (ICU), with attributable mortality of up to 13%, and its etiological diagnosis remains challenging.
Materials and methods: We conducted a multicenter, prospective, observational study within the MULTI-SITA platform to assess the impact on relevant clinical and antimicrobial stewardship outcomes of the use of a molecular syndromic panel (BIOFIRE® FILMARRAY® Pneumonia plus), in addition to a standard approach based on culture. The primary outcome measure was 30-day mortality from VABP onset.
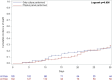
Results: Overall, 237 patients with VABP were included in the study. In multivariable analysis, SOFA score (hazard ratio [HR] 1.13, 95% confidence interval [CI] 1.04–1.22, p = 0.003), previous isolation of carbapenem-resistant Pseudomonas aeruginosa (HR 3.02, 95% CI 1.25–7.32, p = 0.015), and solid neoplasm (HR 2.15, 95% CI 1.12–4.14, p = 0.022) were associated with increased mortality, while no association was registered for the molecular syndromic panel performed (HR 1.07, 95% CI 0.59–1.93, p = 0.825). In secondary analyses, use of the molecular syndromic panel resulted in more events of either de-escalation or initiation of appropriate antibiotic therapy at day 1 from VABP onset in comparison with a standard approach based on culture only (41.3% vs. 27.8%, p = 0.041).
Conclusion: The use of a molecular syndromic panel in patients with VABP was able to impact antibiotic decisions, without an unfavorable effect on mortality. Further study is necessary to assess the long-term effects in terms of antimicrobial stewardship of molecular syndromic panels-based antibiotic treatment decisions.
Supplementary Information: The online version contains supplementary material available at 10.1186/s13054-025-05632-z.
Keywords: Antimicrobial resistance; Antimicrobial stewardship; Rapid diagnosis; Rapid molecular tests; Ventilator-associated pneumonia.
Conflict of interest statement
Declarations. Ethical approval: The MULTI-SITA project was approved by the ethics committee of the coordinating center (Liguria Region Ethics Committee, registry number 390/2020), with a subsequent amendment authorizing the conduct of the RAPID-SITA PHENOTYPES study within the MULTI-SITA project. The other participating centers followed the local ethical committees requirements and started to enroll patients prospectively once activated. Conscious patients at time of enrollment signed an informed consent to participate in the study. A waiver of informed consent for data collection from unconscious patients at the time of enrollment due to severe clinical conditions was obtained within the ethics committee approval, in line with the observational nature of the analyses and in order not to bias research results towards low mortality prejudicing scientific validity. Competing interests: Outside the submitted work, Matteo Bassetti has received funding for scientific advisory boards, travel, and speaker honoraria from Cidara, Gilead, Menarini, MSD, Mundipharma, Pfizer, and Shionogi. Outside the submitted work, Daniele Roberto Giacobbe reports investigator-initiated grants from Pfizer, Shionogi, Menarini, Tillotts Pharma, and Gilead Italia, travel support from Pfizer, and speaker/advisor fees from Pfizer, bioMérieux, Advanz Pharma, Menarini, and Tillotts Pharma. Outside the submitted work, Vincenzo Di Pilato reports travel and speaker honoraria from Arrow Diagnostics. Outside the submitted work, Andrea Cortegiani has received fees for lectures/advisory board membership from Gilead, MSD, Mundipharma, and Pfizer. Outside the submitted work, Gian Maria Rossolini has received research grants for the laboratory, funding for scientific advisory boards and/or speaker engagements from ADA, Advanz Pharma, Alifax, Arrow Diagnostics, bioMérieux, Cepheid, Diesse, Hain Life Sciences, Menarini, Meridian, MSD, Pfizer, Qiagen, Q-linea, Quantamatrix, Quidel, Qvella, SD Biosensor, Seegene, Shionogi, Syncells, Viatris, and Zambon. The other authors report no conflicts of interest.
Figures


References
-
- Koulenti D, Tsigou E, Rello J. Nosocomial pneumonia in 27 ICUs in Europe: perspectives from the EU-VAP/CAP study. Eur J Clin Microbiol Infect Dis. 2017;36(11):1999–2006. - PubMed
-
- Melsen WG, Rovers MM, Groenwold RH, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13(8):665–71. - PubMed
-
- Colaneri M, Montrucchio G, Scaglione G, et al. Incidence, microbiology and mortality of ventilation-associated pneumonia in a large Italian cohort of critically ill patients. Clin Microbiol Infect: Results PROSA proj; 2025. - PubMed
-
- Giacobbe DR, Di Pilato V, Vena A, et al. Interpreting the results of rapid molecular diagnostic tests for carbapenem-resistant Enterobacterales infection: current clinical perspective while waiting for further evidence. Expert Rev Mol Diagn. 2024;24(7):583–90. - PubMed

