Materials Engineering for High Performance and Durability Proton Exchange Membrane Water Electrolyzers
- PMID: 41000102
- PMCID: PMC12458485
- DOI: 10.1021/acsaem.5c01989
Materials Engineering for High Performance and Durability Proton Exchange Membrane Water Electrolyzers
Abstract
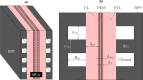
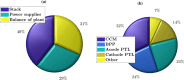
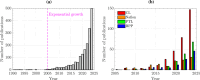
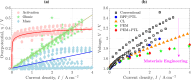
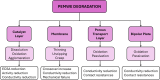
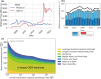
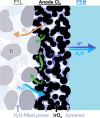
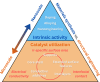
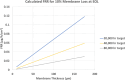
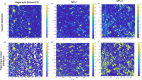
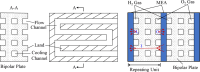
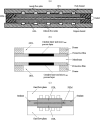
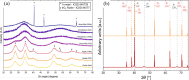
Proton exchange membrane water electrolyzers (PEMWEs) are expected to play a crucial role in the global green energy transition during the 21st century. They provide a versatile and sustainable solution for generating hydrogen with very high purity in combination with renewable energies, such as solar and wind. Despite their promise, PEMWEs face several critical problems, including high costs, performance limitations, and durability challenges, particularly at low iridium (Ir) loading on the anode. Advancing next-generation PEMWEs requires extensive work on materials engineering of all cell components, including the catalyst layer (CL), membrane, porous transport layer (PTL), bipolar plate (BPP), and gasket. This task must be performed with the complementary contribution of different modeling and characterization techniques. This review presents a critical perspective from academia, research centers, and industry, mapping main developments, remaining gaps, and strategic pathways to advance PEMWE technology. A focus is devoted to key aspects, such as operation at low Ir loading, membrane durability, multiscale transport layers, porous and non-porous flow fields, multiphysics modeling, and multipurpose characterization techniques, which are thoroughly discussed. By unifying these topics, this review provides readers with the essential knowledge to grasp current developments and tackle tomorrow's challenges in PEMWE engineering.
Keywords: PEMWE; characterization; design; durability; materials; modeling; performance.
© 2025 The Authors. Published by American Chemical Society.
Figures


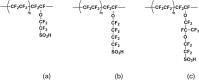


















References
-
- Seck G. S., Hache E., Sabathier J., Guedes F., Reigstad G. A., Straus J., Wolfgang O., Ouassou J. A., Askeland M., Hjorth I., Skjelbred H. I., Andersson L. E., Douguet S., Villavicencio M., Trüby J., Brauer J., Cabot C.. Hydrogen and the decarbonization of the energy system in Europe in 2050: A detailed model-based analysis. Renew. Sustain. Energy Rev. 2022;167:112779. doi: 10.1016/j.rser.2022.112779. - DOI
-
- Pathak P. K., Yadav A. K., Padmanaban S.. Transition toward emission-free energy systems by 2050: Potential role of hydrogen. Int. J. Hydrog. Energy. 2023;48:9921–9927. doi: 10.1016/j.ijhydene.2022.12.058. - DOI
-
- Puertas-Frías C. M., Willson C. S., García-Salaberri P. A.. Design and economic analysis of a hydrokinetic turbine for household applications. Renew. Energy. 2022;199:587–598. doi: 10.1016/j.renene.2022.08.155. - DOI
-
- Yang B., Zhang R., Shao Z., Zhang C.. The economic analysis for hydrogen production cost towards electrolyzer technologies: current and future competitiveness. Int. J. Hydrog. Energy. 2023;48:13767–13779. doi: 10.1016/j.ijhydene.2022.12.204. - DOI
Publication types
LinkOut - more resources
Full Text Sources
