This is a preprint.
Cortical tension as a mechanical barrier to safeguard against premature differentiation during neurogenesis
- PMID: 41000649
- PMCID: PMC12458310
- DOI: 10.1101/2025.09.19.677444
Cortical tension as a mechanical barrier to safeguard against premature differentiation during neurogenesis
Abstract
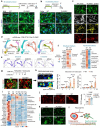
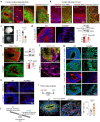
Neuronal differentiation requires coordinated gene reprogramming and morphodynamic remodeling. How mechanical forces integrate with nuclear gene programs during neurogenesis remains unresolved. Here, we identify cortical tension as a mechanical barrier that safeguards against premature neuronal differentiation. Deletion of Plexin-B2, a guidance receptor controlling actomyosin contractility, lowers this barrier, enabling neurite outgrowth and accelerating neuronal lineage commitment. We show that coupling of extrinsic differentiation cues with intrinsic morphodynamics is essential for stabilizing neuronal fate and that cortical barrier and epigenetic barrier act in concert to regulate developmental timing. In cerebral organoids, Plexin-B2 ablation triggered premature cell-cycle exit and differentiation, resulting in progenitor pool depletion and neuroepithelial disorganization, phenotypes echoing intellectual disability in patients with rare pathogenic PLXNB2 variants. Our studies demonstrate that cortical tension functions as mechano-checkpoint that regulates the onset of neurogenesis. Lowering this barrier may provide a strategy to accelerate induced neuron generation and maturation for CNS disease modeling.
Keywords: Cerebral organoid; Cortical barrier; Corticogenesis; Induced neuron; Neurodevelopment; Neuronal differentiation; Plexin-B2.
Conflict of interest statement
Competing interests R.H.F, H.Z., S.K., X.L, and Z.Y. are named inventors of technology related to anti-Plexin-B2 antibodies. This technology is the subject of a patent application filed by the Icahn School of Medicine at Mount Sinai. Other authors declare that they have no competing interests.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
