This is a preprint.
Evolutionary antifragile therapy
- PMID: 41000654
- PMCID: PMC12458151
- DOI: 10.1101/2025.09.11.675645
Evolutionary antifragile therapy
Abstract
A population of cells within a tumor can be described as antifragile (the opposite of fragile) if they derive a benefit from fluctuations or perturbations in environmental conditions induced by treatment. We hypothesized that treatment fluctuations may either promote (antifragile tumor) or inhibit (fragile tumor) the evolution of treatment resistance to targeted therapies. Analysis of the convexity of dose response curves provides a direct prediction of response to prescribed fluctuations in treatment. Theory predicts that continuous treatment protocols (i.e. zero prescribed fluctuations in treatment) will outperform uneven protocols when dose response is convex. We apply this theory to predict in vivo response to targeted therapy and validate these predictions by experimentally testing high/low intermittent dosing (uneven dosing) and continuous dosing (even dosing) schedules. Convexity (and its inverse, concavity) explains two phenomena: dose response is a convex (fragile) function but resistance onset rate is a concave (antifragile) function. Thus, we design, propose, and validate alternative treatment schedules which maximize response while maintaining prolonged sensitivity to treatment. These analyses provide supporting evidence that fluctuations alter the evolutionary trajectory of tumors in response to targeted therapy, even without altering the cumulative dose. This insight is used to design alternative, switching treatment protocols to limit the evolution of resistance, a process which we call evolutionary antifragile therapy.
Conflict of interest statement
Competing Interests The authors declare no competing interests.
Figures




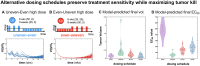


References
-
- Sasaki T., Rodig S. J., Chirieac L. R. & Jänne P. A. The biology and treatment of eml4-alk non-small cell lung cancer. European journal cancer 46, 1773–1780 (2010).
-
- Vander Velde R. et al. Resistance to targeted therapies as a multifactorial, gradual adaptation to inhibitor specific selective pressures. Nature communications 11, 2393 (2020).
-
- Tracey A. FDA’s Brian Booth: “We need to reconsider our approach to dose selection”. The Cancer Letter (2022).
-
- Taleb N. N. Antifragile: Things that gain from disorder, vol. 3 (Random House Incorporated, 2012).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
