This is a preprint.
CNS-Tau Specific Antibodies Illuminate Disease Signatures Across Tauopathies
- PMID: 41000694
- PMCID: PMC12458136
- DOI: 10.1101/2025.09.14.676119
CNS-Tau Specific Antibodies Illuminate Disease Signatures Across Tauopathies
Abstract
Background: Alternative splicing of the MAPT gene produces distinct tau isoforms in the central and peripheral nervous systems (CNS and PNS), yet their respective biological and pathological roles remain poorly understood. Recent studies suggest that CNS-tau may play a key role in amyloid-β associated neurodegeneration in Alzheimer's disease (AD), but the absence of isoform-specific tools has limited both mechanistic insight and biomarker development. We aimed to develop and validate CNS-tau-specific monoclonal antibodies and assess their utility in neuropathology and fluid-based biomarker assays across AD and primary tauopathies.
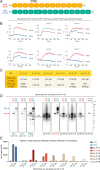
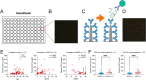
Methods: We generated six recombinant rabbit monoclonal antibodies targeting a CNS-tau-specific sequence encoded by exons 4 and 5. Specificity and affinity were evaluated via biolayer interferometry, immunoblotting, and tau-expressing HEK293 models. The lead clone, LL-T-1-1, was tested in postmortem brain sections from AD (n = 23), progressive supranuclear palsy (PSP, n = 3), and corticobasal degeneration (CBD, n = 3). A second clone, LL-T-1-5, was optimized for use in plasma assays via an ultrasensitive nanoneedle platform, LoD < 1 pg/ml.
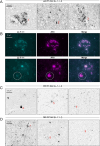
Results: LL-T-1-1 showed nanomolar affinity for CNS-tau and no cross-reactivity with PNS-tau. It selectively labeled dystrophic neurites in AD and all hallmark tau lesions in PSP and CBD without antigen retrieval. LL-T-1-5-based plasma assays revealed CNS-tau levels significantly correlated with cognitive scores (MMSE and QDRS) and differentiated impaired from unimpaired individuals.
Conclusions: CNS-tau-specific antibodies LL-T-1-1 and LL-T-1-5 provide new tools for neuropathology and fluid biomarker development across tauopathies.
Keywords: Alzheimer’s disease; CNS-specific tau isoform; neurodegenerative disease.
Conflict of interest statement
Declaration of Interests Luke Slominski and Qimin Quan are employees of NanoMosaic, Inc. DJS is a director of Prothena Biosciences and an ad hoc consultant to Eisai and Roche. All other authors have nothing to disclose.
Figures

References
-
- Liu L, Kwak H, Lawton TL, et al. An ultra-sensitive immunoassay detects and quantifies soluble Aβ oligomers in human plasma. Alzheimer’s & Dementia. 2022;18(6):1186–1202. doi: 10.1002/alz.12457 - DOI
-
- Bellier J, Cai Y, Alam SM, et al. Uncovering elevated tau TPP motif phosphorylation in the brain of Alzheimer’s disease patients. Alzheimer’s & Dementia. 2024;20(3):1573–1585. doi: 10.1002/alz.13557 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
