This is a preprint.
Gastrointestinal delivery of mRNA lipid nanoparticles selectively targets the pancreas
- PMID: 41000865
- PMCID: PMC12458220
- DOI: 10.1101/2025.09.15.676190
Gastrointestinal delivery of mRNA lipid nanoparticles selectively targets the pancreas
Abstract
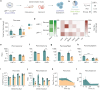
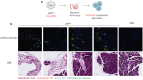
Lipid nanoparticles (LNPs) administered parenterally often show poor localization to the gastrointestinal (GI) tract and pancreas. In addition, patients typically prefer orally administered drugs to those given intravenously. We therefore investigated whether GI delivery, achievable via device mediated microneedle injections applied to buccal, gastric, small intestinal, colonic, or rectal tissues, could simultaneously enhance LNP delivery to the GI and pancreas while avoiding intravenous administration. Using a combined approach of formulation optimization and GI delivery site screening, we found that cationic SM-102 LNPs delivered gastrically achieved 7-fold higher pancreas delivery in rodents than intravenous neutral SM-102 LNPs. With dose optimization, gastric LNPs achieved 6000-fold greater pancreas to liver targeting ratios than intravenous LNPs. These results suggest GI microneedle administration can reprogram LNP biodistribution, thereby expanding therapeutic opportunities for both local and systemic nucleic acid delivery.
Conflict of interest statement
Competing Interests: A.M.A., R.G., A.A., and J.E.D. are inventors on provisional patents describing the technology presented in this manuscript. J.E.D. advises Readout Capital, Edge Animal Health and Nava Therapeutics. A.A.’s full list of competing interests can be found at sites.google.com/view/alex-abramson-coi. The other authors declare no competing interests.
Figures

References
-
- FDA, “FDA Approves Drug to Reduce Triglycerides in Adult Patients with Familial Chylomicronemia Syndrome,” FDA. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-drug-redu....
-
- FDA, “MNEXSPIKE,” FDA. https://www.fda.gov/vaccines-blood-biologics/mnexspike.
-
- Huang X., et al. , “Oral Delivery of Liquid mRNA Therapeutics by an Engineered Capsule for Treatment of Preclinical Intestinal Disease,” Sci. Transl. Med. 17, eadu1493 (2025). - PubMed
-
- Boirivant M., et al. , “Inhibition of Smad7 with a Specific Antisense Oligonucleotide Facilitates TGF-beta1-Mediated Suppression of Colitis,” Gastroenterology 131 (2006): 1786–1798. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
