Schwann cells in the inner ear: development, disease, and regeneration
- PMID: 41001135
- PMCID: PMC12457403
- DOI: 10.3389/fncel.2025.1662274
Schwann cells in the inner ear: development, disease, and regeneration
Abstract
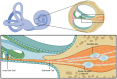
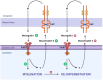
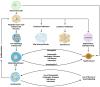
Schwann cells are classically known as the constituent supporting cells of the peripheral nervous system. Beyond the scope of merely myelinating axons of the more saliently known neurons, Schwann cells comprise the majority of peripheral nervous system tissue. Through the lens of the inner ear, additional properties of Schwann cells are becoming elucidated. Therein, the process of myelin formation in development is more aptly understood as a homeostatic oscillation of differentiation status. Perpetual interaction between neural and non-neural cells of the inner ear maintains an intricate balance of guidance, growth, and maturation during development. In disease, aberration to Schwann cell myelination contributes to sensorineural hearing loss in conditions such as Guillain-Barre Syndrome and Charcot-Marie-Tooth disease, and tumorigenic over proliferation of Schwann cells defines vestibular schwannomas seen in neurofibromatosis type 2. Schwann cells demonstrate plasticity during oscillations between differentiation and dedifferentiation, a property that is now being leveraged in efforts to regenerate lost neurons. Emerging strategies of reprogramming, small molecule modulation, and gene therapy suggest that Schwann cells could serve as progenitor cells for regenerated neurons. Understanding the duality of Schwann cells in pathology and repair could transform the approach to treating sensorineural hearing loss.
Keywords: NF2; Schwann cells; cochlea; glia; inner ear; myelin; regeneration; schwannoma.
Copyright © 2025 Montigny and Kempfle.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

