Stabilizing a Native Fold of Alpha-Synuclein with Short Helix-Constrained Peptides
- PMID: 41001629
- PMCID: PMC12458024
- DOI: 10.1021/jacsau.5c00694
Stabilizing a Native Fold of Alpha-Synuclein with Short Helix-Constrained Peptides
Abstract
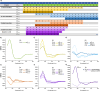
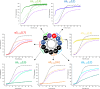
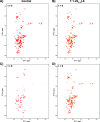
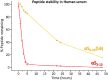
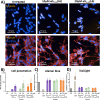
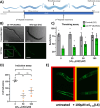
Preventing the aggregation of α-synuclein (αS) into toxic oligomers and conformers is a major therapeutic goal in conditions such as Parkinson's disease and Lewy body dementia. However, the large intracellular protein-protein interfaces within such aggregates make this a challenging target for small molecule approaches or biologics, which often lack cell permeability. Peptides occupy a suitable middle ground and are increasingly being explored as preventative treatments. We previously showed that the N-terminal lipid binding region (αS1-25) inhibits αS aggregation. Building on this, we designed a series of N- and C-terminal truncations to systematically reduce the peptide length, enabling a 56% downsizing (i.e., truncating 92% of the full-length αS protein), to identify the smallest functional unit capable of binding αS and potently blocking its aggregation and toxicity. We next introduced seven systematic i → i + 4 helix constraints to assess impact on (i) α-helicity, (ii) aggregation inhibition, (iii) serum stability, (iv) neuronal uptake, and (v) phenotypic rescue. This work maps key amphipathic features and identifies residues that are critical for αS engagement and inhibitory activity. The most effective helix-constrained peptide, αS2-12(L6), showed marked improvements across all metrics and represents a strong candidate for further therapeutic development.
Keywords: Parkinson’s disease; amyloid aggregation; lipid induced aggregation; lipid vesicles; peptide.
© 2025 The Authors. Published by American Chemical Society.
Figures





References
-
- Meade R. M., Allen S. G., Williams C., Tang T. M. S., Crump M. P., Mason J. M.. An N-terminal alpha-synuclein fragment binds lipid vesicles to modulate lipid-induced aggregation. Cell Rep. Phys. Sci. 2023;4(9):101563. doi: 10.1016/j.xcrp.2023.101563. - DOI
-
- Dehay B., Bourdenx M., Gorry P., Przedborski S., Vila M., Hunot S., Singleton A., Olanow C. W., Merchant K. M., Bezard E.. et al. Targeting alpha-synuclein for treatment of Parkinson’s disease: mechanistic and therapeutic considerations. Lancet Neurol. 2015;14(8):855–866. doi: 10.1016/S1474-4422(15)00006-X. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
