Recent Advances in Microfluidic Biofuel Cells
- PMID: 41002366
- PMCID: PMC12467921
- DOI: 10.3390/bios15090627
Recent Advances in Microfluidic Biofuel Cells
Abstract
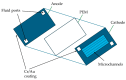
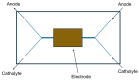
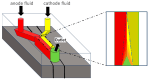
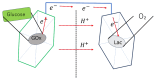
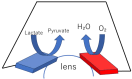
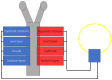
Traditionally, fuel cells operate by using small fuel molecules such as hydrogen and methanol to produce energy, water, and carbon dioxide. Enzyme biofuel cells use enzymes rather than precious metals as electrode catalysts. In recent years, enzyme-immobilized electrodes have been developed by combining enzyme biofuel cells with microfluidic technology to improve the efficiency and performance of fuel cells. In this review, we will provide an overview and describe the current status of recent enzyme biofuel cells, microfluidic technology, and their applications to microfluidic fuel cells.
Keywords: (bio)fuel cells; electron transfer; enzyme immobilization; mediators; microfluidic fuel cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Estrada-Osorio D.V., Escalona-Villalpando R.A., Gurrola M.P., Chaparro-Sánchez R., Rodríguez-Morales J.A., Arriaga L.G., Ledesma-García J. Abiotic, Hybrid, and Biological Electrocatalytic Materials Applied in Microfluidic Fuel Cells: A Com-prehensive. ACS Meas. Sci. Au. 2024;4:25–41. doi: 10.1021/acsmeasuresciau.3c00044. - DOI - PMC - PubMed
-
- Falk M., Blum Z., Shleev S. Direct electron transfer based enzymatic fuel cells. Electrochem. Acta. 2012;82:191–202. doi: 10.1016/j.electacta.2011.12.133. - DOI
-
- Luz R.A.S., Pereira A.R., de Souza J.C.P., Sales F.C.P.F., Crespilho F.N. Enzyme Biofuel Cells: Thermodynamics, Kinetics and Challenges in Applicability. ChemElectroChem. 2014;1:1751–1771. doi: 10.1002/celc.201402141. - DOI
-
- Khalil M., Ahmad F., Khan M.I., Shanableh A., Taj A.B., Rao K.A., Voskressensky L.G., Luque R. A critical review of biofuel cell cathodes. Biofuels Bioprod. Bioref. 2025 doi: 10.1002/bbb.2754. Early View . - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

