Interaction Between Human Skeletal and Mesenchymal Stem Cells Under Physioxia Enhances Cartilage Organoid Formation: A Phenotypic, Molecular, and Functional Characterization
- PMID: 41002388
- PMCID: PMC12468709
- DOI: 10.3390/cells14181423
Interaction Between Human Skeletal and Mesenchymal Stem Cells Under Physioxia Enhances Cartilage Organoid Formation: A Phenotypic, Molecular, and Functional Characterization
Abstract
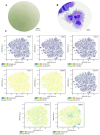
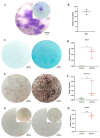
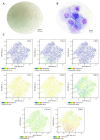
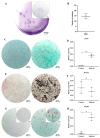
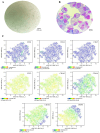
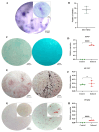
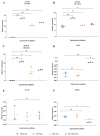
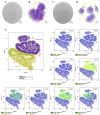
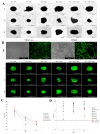
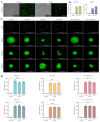
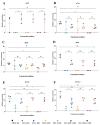
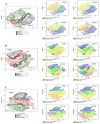
Articular cartilage regeneration remains a major challenge due to its limited self-repair capacity. Bone marrow-derived skeletal stem cells (SSCs) and mesenchymal stem cells (MSCs) are promising candidates for cartilage engineering, although they differ in their chondrogenic potential. This study explored whether co-culturing SSCs and MSCs in three-dimensional (3D) organoid systems under cartilage physioxia (5% O2) and chondrogenic induction could improve cartilage tissue formation. SSCs, MSCs, and SSC-MSC co-cultures were characterized for morphology, phenotype, and differentiation capacity. Organoids were generated and cultured for 10 days, followed by analysis of morphology, viability, gene expression (SOX9, RUNX2, ACAN, COL2A1, COL10A1, PRG4, and PDPN), chondrocyte-associated antigens (CD44, CD105, CD146, and PDPN), and cartilage ECM proteins (aggrecan, collagen types I, II, and X, and PRG4). SSCs showed robust chondrogenic and osteogenic potential, while MSCs exhibited a balanced multipotency. Co-culture-derived organoids enhanced chondrogenesis and reduced adipogenesis, with higher expression of cartilage-specific ECM and lower hypertrophic marker levels. These findings highlight the functional synergy between SSCs and MSCs in co-culture, promoting the formation of stable, cartilage-like structures under physioxia. The approach offers a promising strategy for generating preclinical models and advancing regenerative therapies for hyaline cartilage repair.
Keywords: cartilage organoids; chondrogenesis; mesenchymal stem cells; physioxia; skeletal stem cells.
Conflict of interest statement
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures

References
-
- Belluzzi E., Todros S., Pozzuoli A., Ruggieri P., Carniel E.L., Berardo A. Human Cartilage Biomechanics: Experimental and Theoretical Approaches towards the Identification of Mechanical Properties in Healthy and Osteoarthritic Conditions. Processes. 2023;11:1014. doi: 10.3390/pr11041014. - DOI
-
- Householder N.A., Raghuram A., Agyare K., Thipaphay S., Zumwalt M. A Review of Recent Innovations in Cartilage Regeneration Strategies for the Treatment of Primary Osteoarthritis of the Knee: Intra-Articular Injections. Orthop. J. Sports Med. 2023;11:23259671231155950. doi: 10.1177/23259671231155950. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

