CO2 Adsorption by Amino-Functionalized Graphene-Silica Gels
- PMID: 41002476
- PMCID: PMC12469925
- DOI: 10.3390/gels11090702
CO2 Adsorption by Amino-Functionalized Graphene-Silica Gels
Abstract
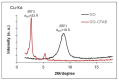
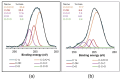
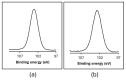
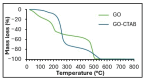
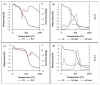
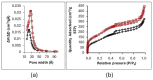
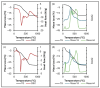
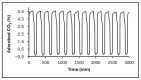
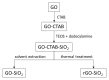
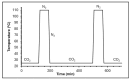
This work evaluates the CO2-adsorption relevance and cycling stability of graphene oxide-silica (GO-SiO2) and reduced graphene oxide-silica (rGO-SiO2) gels after amine functionalization, demonstrating high-capacity retention under repeated adsorption-desorption cycles: rGO-SiO2-APTMS retains ≈96.3% of its initial uptake after 50 cycles, while GO-SiO2-APTMS retains ≈90.0%. The use of surfactants to control the organization of inorganic and organic molecules has enabled the development of ordered mesostructures, such as mesoporous silica and organic/inorganic nanocomposites. Owing to the outstanding properties of graphene and its derivatives, synthesizing mesostructures intercalated between graphene sheets offers nanocomposites with novel morphologies and enhanced functionalities. In this study, GO-SiO2 and rGO-SiO2 gels were synthesized and characterized by X-ray diffraction (XRD), differential scanning calorimetry (DSC), thermogravimetric analysis (TG), mass spectrometry (MS), N2 adsorption-desorption isotherms, and transmission electron microscopy (TEM). The resulting materials exhibit a laminar architecture, with mesoporous silica domains grown between graphene-based layers; the silica contents are 83.6% and 87.6%, and the specific surface areas reach 446 and 710 m2·g-1, respectively. The laminar architecture is retained regardless of the surfactant-removal route; however, in GO-SiO2 obtained by solvent extraction, a fraction of the surfactant remains partially trapped. Together with their high surface area, hierarchical porosity, and amenability to surface functionalization, these features establish amine-grafted graphene-silica gels, particularly rGO-SiO2-APTMS, as promising CO2-capture adsorbents.
Keywords: CO2 capture; amine functionalization; graphene; graphene oxide; mesostructure; silica gels; sol–gel; surfactant removal.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Singh V., Joung D., Zhai L., Das S., Khondaker S.I., Seal S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011;56:1178–1271. doi: 10.1016/j.pmatsci.2011.03.003. - DOI
-
- Wei L., Lu W., Wei H., Chen C., Hou Z. Porous sandwich-like silica/graphene nanocomposites obtained via templating of porous silica with CTAB in the gallery region of graphene oxide. Microporous Mesoporous Mater. 2017;241:58–65.
-
- Liu L., Zou G., Yang B., Luo X., Xu S. Amine-functionalized mesoporous silica @ reduced graphene sandwichlike structure composites for CO2 adsorption. ACS Appl. Nano Mater. 2018;1:4695–4702. doi: 10.1021/acsanm.8b00943. - DOI
-
- Wang Z.M., Wang W., Coombs N., Soheilnia N., Ozin G.A. Graphene oxide-periodic mesoporous silica sandwich nanocomposites with vertically oriented channels. ACS Nano. 2010;4:7437–7450. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

