CAR-T Access Disparities for Multiple Myeloma in the Midwest: A Social Determinants of Health Perspective
- PMID: 41002565
- PMCID: PMC12468812
- DOI: 10.3390/curroncol32090495
CAR-T Access Disparities for Multiple Myeloma in the Midwest: A Social Determinants of Health Perspective
Abstract
Background: Multiple Myeloma (MM) is the most common type of blood cancer among black individuals. CAR-T therapy is crucial, but often inaccessible to many black patients and those from underserved communities. The University of Kansas Health System administers over 100 CAR-T treatments annually and aims to evaluate barriers to CAR-T therapy access related to the social determinants of health in the Midwest area.
Methods: This study examined patients with MM referred for CAR-T therapy from January 2021 to December 2023, assessing how race, socioeconomic status, and insurance influenced eligibility for leukapheresis. Data on income and travel were gathered from the 2022 US Census and analyzed using R software.
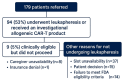
Results: The study included 271 referrals for MM CAR-T therapy involving 179 patients, with a median age of 66 years (51% male).
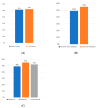
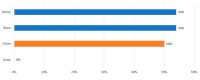
Demographics: 80% white, 16% black, 2.2% other races, 1.8% Asian, with a median income of $70,644. Nearly half lived more than 30 min from the center (Mainly from Kansas, Missouri and Nebraska). Apheresis rates were similar across racial groups: 54% for whites, 54% for blacks, and 50% for others, while none of the three Asian patients proceeded. Nine patients (5%) could not proceed because of caregiver or insurance barriers, and cell collection rates were comparable regardless of distance (34% vs. 35%).
Conclusion: This study showed that black representation in CAR-T access matches local demographics, indicating less disparity among minorities. Unlike national reports, distance, income, and insurance do not significantly affect access, suggesting the need for a national study on the social determinants impacting CAR-T access for multiple myeloma.
Keywords: CAR-T therapy; multiple myeloma; social determinants of health.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Berdeja J.G., Madduri D., Usmani S.Z., Jakubowiak A., Agha M., Cohen A.D., Stewart A.K., Hari P., Htut M., Lesokhin A.M., et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet. 2021;398:314–324. doi: 10.1016/S0140-6736(21)00933-8. - DOI - PubMed
-
- Ahmed N., Shahzad M., Shippey E., Ganatra S., Wetzler M., McCarthy P.L., Reagan J.L., Al-Homsi A.S., Ghosh N., Lazarus H.M., et al. Socioeconomic and Racial Disparity in Chimeric Antigen Receptor T Cell Therapy Access. Transplant. Cell. Ther. 2022;28:358–364. doi: 10.1016/j.jtct.2022.04.008. - DOI - PubMed
-
- Chung A., Shafrin J., Vadgama S., Hurley K., Perales M.-A., Alsfeld L.C., Muthukrishnan S., Patel A.R., Shah G.L., Maziarz R.T. Inequalities in CAR T-Cell Therapy Access for US Patients with Relapsed/Refractory DLBCL: A SEER-Medicare Data Analysis. Blood Adv. 2025 doi: 10.1182/bloodadvances.2024015634. - DOI - PubMed
-
- Leahy A.B., Newman H., Li Y., Liu H., Myers R., DiNofia A., Dolan J.G., Callahan C., Baniewicz D., Devine K., et al. CD19-targeted chimeric antigen receptor T-cell therapy for CNS relapsed or refractory acute lymphocytic leukaemia: A post-hoc analysis of pooled data from five clinical trials. Lancet Haematol. 2021;8:e711–e722. doi: 10.1016/S2352-3026(21)00238-6. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical