Isavuconazole Therapy for Patients with Hematologic Diseases and Hematopoietic Cell Transplantation with and Without Breakthrough Invasive Fungal Infections
- PMID: 41003194
- PMCID: PMC12471279
- DOI: 10.3390/jof11090648
Isavuconazole Therapy for Patients with Hematologic Diseases and Hematopoietic Cell Transplantation with and Without Breakthrough Invasive Fungal Infections
Abstract
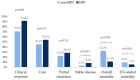
There are no data available on the effectiveness and safety of isavuconazole (ISA) for treating breakthrough invasive fungal infections (bIFIs). A retrospective and prospective cohort study was conducted between January 2020 and March 2025 in 13 centers in Argentina. Hematologic diseases (HD) and hematopoietic cell transplantation (HCT) patients who received ISA for IFI were included and followed for 12 weeks. Patients with proven and probable bIFIs and non-bIFIs were compared. One hundred and sixty-three patients were included. IFIs were classified as proven (13.5%), probable (26.9%) and possible (59.5%). Among 66 proven and probable IFIs, 53% were bIFIs, with aspergillosis and mucormycosis being the most common. Twenty-three (34.8%) patients had acute myelogenous leukemia, and 40.9% had received HCT. Forty-eight (72.7%) patients experienced neutropenia, with a median duration of 26 days (interquartile range [IQR] 16-44). Fluconazole and posaconazole were the most frequently received antifungal prophylaxis. ISA was prescribed as first-line therapy in 31 (46.9%) patients. The other 35 received ISA as a continuation therapy, mainly as a step-down therapy after liposomal amphotericin B. Four (6.1%) patients developed adverse effects, and one discontinued ISA. The 90-day overall clinical response between patients with bIFI vs. non-bIFI was 91.4% vs. 70.9% (p = 0.052). The 90-day overall and IFI-related mortality rates were, respectively, 11.4% vs. 32.3% (p = 0.068) and 5.7% vs. 9.7% (p = 0.659). The study data evidence ISA effectiveness and safety for the treatment of HD and HCT patients with and without bIFIs.
Keywords: breakthrough invasive fungal infections; hematologic diseases; hematopoietic cell transplantation; isavuconazole therapy.
Conflict of interest statement
F.H. has participated in advisory boards and/or received speaker honoraria and grants from Gilead, Knight Therapeutics, Merck, Sharp & Dohme (MSD), SteinCares, Biomerieux, Rochem Biocare, TEVA, TAKEDA, and Pfizer. D.T. has participated in advisory boards and/or received speaker honoraria from Gilead, Knight Therapeutics, MSD, Pfizer and GlaxoSmithKline. G.M. has participated in advisory boards and/or received speaker honoraria from Knight Therapeutics. R.J. has participated in advisory boards and/or received speaker honoraria from Knight Therapeutics and Pfizer. J.A. has participated in advisory boards and/or received speaker honoraria from Knight Therapeutics, Gilead and Pfizer. All other authors report no potential conflicts of interest.
Figures
References
-
- Girmenia C., Raiola A.M., Piciocchi A., Algarotti A., Stanzani M., Cudillo L., Pecoraro C., Guidi S., Iori A.P., Montante B., et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation: A prospective study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO) Biol. Blood Marrow Transplant. 2014;20:872–880. doi: 10.1016/j.bbmt.2014.03.004. - DOI - PubMed
-
- Busca A., Passera R., Maffini E., Festuccia M., Brunello L., Dellacasa C.M., Aydin S., Frairia C., Manetta S., Butera S., et al. Hematopoietic cell transplantation comorbidity index and risk of developing invasive fungal infections after allografting. Bone Marrow Transplant. 2018;53:1304–1310. doi: 10.1038/s41409-018-0161-1. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources