Development of a Patient-Centered Outcome Tool for Blepharospasm: A Stepwise Modified Delphi Study
- PMID: 41003519
- PMCID: PMC12474189
- DOI: 10.3390/toxins17090455
Development of a Patient-Centered Outcome Tool for Blepharospasm: A Stepwise Modified Delphi Study
Abstract
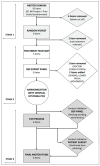
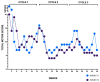
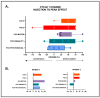
Blepharospasm (BSP) is characterized by excessive orbicularis oculi muscle activity leading to abnormal blinking and involuntary eyelid closure. Botulinum neurotoxin (BoNT) injections are the main treatment for BSP, but they only partially and transiently relieve symptoms, leading to a waxing and waning therapeutic response. A patient-centered outcome (PCO) tool that measures BSP symptoms in a simple and efficient way could inform the development of better treatments. Using a stepwise modified Delphi approach, potential PCO items were first identified using the Dystonia Coalition Database with data from over 200 individuals with BSP who had provided responses to existing clinical assessment scales. These items were then analyzed for contribution to overall severity using a Random Forests approach, and redundant items were merged and revised in a series of iterative meetings with a specialist panel along with input from patient advocacy group representatives and focus groups. An online survey was conducted with 330 individuals with BSP to validate and verify the items' relevance. Finally, the specialist panel provided content validity ratio, which was repeated until it showed good agreement for relevance and clarity of all items. In the end, an easy-to-use PCO tool designed for smartphones and tablets containing 17 items covering three symptom domains (motor, disability, and psychosocial/quality of life) was created. This novel PCO tool for BSP may be used to characterize the cyclical response that an individual patient experiences from BoNT treatments and provide a vital tool for future investigations of longer-acting BoNT preparations or adjunctive therapies.
Keywords: blepharospasm; botulinum neurotoxin; patient-centered outcomes; self-assessments; symptom monitoring; treatment tracking.
Conflict of interest statement
BDB has received research grant support from the Dystonia Coalition, the Dystonia Medical Research Foundation, and the Benign Essential Blepharospasm Research Foundation; serves on the Medical and Scientific Advisory Council of the Dystonia Medical Research Foundation; is the director of the Medical Advisory Board of the Benign Essential Blepharospasm Research Foundation and a member of the National Spasmodic Torticollis Association; and has also served as a consultant for the Dystonia Medical Research Foundation. ADH has received research support from Argenx and served as an advisor for Amgen and Catalyst Pharmaceuticals. ARH has received research support and served as a consultant for Viridian, served as a consultant and received speaker fees from Amgen, and has served as a consultant for Argenx. MH serves as a member of medical advisory boards for the Dystonia Medical Research Foundation and the Benign Essential Blepharospasm Research Foundation, as well as for Brainsway, QuantalX, and VoxNeuro, and receives patent license fees from Brainsway (H-COIL). JB and CH serve on the Board of Directors for the Benign Essential Blepharospasm Research Foundation. JH serves on the Board of Directors for the Dystonia Medical Research Foundation. HAJ has received grant support from the NIH, including being the principle investigator for the Dystonia Coalition, which has received the majority of its support for its research projects through the NIH (NS116025 and NS065701 from the NINDS and TR001456 from the Office of Rare Diseases Research at the National Center for Advancing Translational Sciences), private philanthropic organizations (Cure Dystonia Now and Lesch-Nyhan Syndrome Children’s Research Foundation), and industry (Abbvie, Addex, Aeon, Motric, Sage, Ipsen, and Jazz); has served on advisory boards or as a consultant for the NIH (CREATE Bio DSMB) and industry (Abbvie, Addex, Ipsen, Merz, and Vima); and has received stipends for administrative work from the International Parkinson’s Disease and Movement Disorders Society and served on scientific advisory boards for the Benign Essential Blepharospasm Research Foundation and the Dystonia Medical Research Foundation. SPR has received grant support from the National Institutes of Health, the Department of Defense, and the Michael J Fox Foundation, has received honoraria from the International Parkinson and Movement Disorders Society and the American Academy of Neurology, and serves on the Medical Advisory Board of the Benign Essential Blepharospasm Research Foundation. The remaining authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. No funders had a role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Defazio G., Jinnah H.A., Berardelli A., Perlmutter J.S., Berkmen G.K., Berman B.D., Jankovic J., Bäumer T., Comella C., Cotton A.C., et al. Diagnostic Criteria for Blepharospasm: A Multicenter International Study. Park. Relat. Disord. 2021;91:109–114. doi: 10.1016/j.parkreldis.2021.09.004. - DOI - PMC - PubMed
-
- Scorr L.M., Cho H.J., Kilic-Berkmen G., McKay J.L., Hallett M., Klein C., Baumer T., Berman B.D., Feuerstein J.S., Perlmutter J.S., et al. Clinical Features and Evolution of Blepharospasm: A Multicenter International Cohort and Systematic Literature Review. Dystonia. 2022;1:10359. doi: 10.3389/dyst.2022.10359. - DOI - PMC - PubMed
-
- Berman B.D., Groth C.L., Sillau S.H., Pirio Richardson S., Norris S.A., Junker J., Brüggemann N., Agarwal P., Barbano R.L., Espay A.J., et al. Risk of Spread in Adult-Onset Isolated Focal Dystonia: A Prospective International Cohort Study. J. Neurol. Neurosurg. Psychiatry. 2020;91:314–320. doi: 10.1136/jnnp-2019-321794. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

