18F-BMS-986229 PET imaging of tumor PD-L1 expression in glioblastoma patients
- PMID: 41003913
- PMCID: PMC12474851
- DOI: 10.1186/s13550-025-01283-x
18F-BMS-986229 PET imaging of tumor PD-L1 expression in glioblastoma patients
Abstract
Background: Neurooncologists urgently need biomarkers that can optimize the clinical development of PD-L1 targeted immunotherapy in the treatment of glioblastoma. This study evaluated PD-L1 targeted tumor imaging by positron emission tomography (PET) in glioblastoma patients, using the novel macrocyclic peptide radiotracer 18F-BMS-986229.
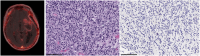
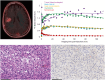
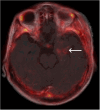
Results: Twelve adult postsurgical glioblastoma patients underwent brain PET imaging 1-hour post injection 190±20 MBq of 18F-BMS-986229. In a subset of patients, dynamic PET scans were obtained for pharmacokinetic modeling in tumors and normal tissues. Tracer kinetics in both tumor sites and normal tissues were well described by a reversible 1-tissue compartment model. Tumor sites demonstrated 18F-BMS-986229 tracer-avidity (SUV = 1.1 ± 0.4; range, 0.6-1.7) in 10 of 12 cases, with negligible tracer-avidity in normal brain structures. Tumor avidity for 18F-BMS-986229 on PET was spatially independent of tumor contrast-enhancement on magnetic resonance imaging, indicating that tracer-binding at tumor site was not dependent upon blood-brain barrier breakdown. The observed tumor site low tracer-uptake paralleled low immunohistochemical PD-L1 expression in resected tumors, with no correlations between standardized uptake value versus tumor MGMT methylation, PTEN oncogenic mutation status, tumor mutation burden, or patient overall survival.
Conclusion: This pilot study demonstrates the feasibility of characterizing tumor sites in glioblastoma patients by PD-L1-targeted PET imaging with18F-BMS-986229, even in patients with low tumor PD-L1 expression. We hypothesize that 18F-BMS-986229 PET can improve the pharmacometrics of PD-L1-targeted therapy trials.
Trial registration number: NCT02617589. Trial Registration Date: December 1st, 2015.
Keywords: 18F-BMS-986229; Glioblastoma; PD-L1 expression; Pharmacokinetic modeling.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Institutional Review Board (Protocol #16–078, ClinicalTrials.gov Identifier: NCT02617589) and performed under an investigational new drug application approved by U.S. Food and Drug Administration. The analysis was conducted following the principles outlined in the Declaration of Helsinki. Patient clinical information was collected anonymously from electronic medical records. Consent for publication: All patients provided written informed consent. Competing interests: IKM reports receiving personal fees (advisory board services) from Agios, Black Diamond Therapeutics, Debiopharm Group, Erasca, Novartis, Prelude Therapeutics, Roche Therapeutics, Servier Pharmaceuticals, Voyager. IKM reports receiving grants from General Electric and Puma Biotechnology. No potential conflicts of interest relevant to this article exist.
Figures

References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

