Reinforced plant-derived lipid nanoparticles for oral precise epigenome editing in colonic diseases
- PMID: 41004579
- PMCID: PMC12466914
- DOI: 10.1126/sciadv.adw9275
Reinforced plant-derived lipid nanoparticles for oral precise epigenome editing in colonic diseases
Abstract
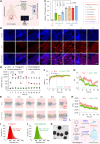
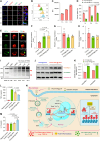
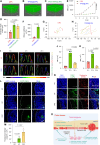
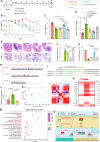
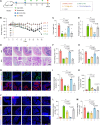
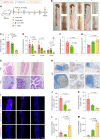
The clinical application of CRISPR-Cas9 remains limited by delivery challenges, particularly for oral administration. Lysine-specific demethylase 1 (Lsd1) plays a key role in colonic inflammation and tumorigenesis. Here, we developed an oral genome-editing platform (TPGS-RNP@LNP), where Lsd1-targeting ribonucleoproteins (RNPs) were encapsulated in mulberry leaf lipid nanoparticles (LNPs) and formulated with d-α-tocopherol polyethylene glycol succinate (TPGS). TPGS reinforced the lipid bilayer of LNPs, enhanced gastrointestinal stability, and facilitated colonic mucus penetration. Upon the galactose receptor-mediated endocytosis of TPGS-RNP@LNPs by macrophages, their fusion with the endosomal membrane and the presence of nuclear localization signals ensured the nuclear delivery of RNPs. TPGS-RNP@LNPs achieved 59.7% Lsd1 editing efficiency in macrophages, surpassing the commercial CRISPRMAX (43.0%). Oral TPGS-RNP@LNPs promoted H3K4 methylation to modulate epigenetic states, achieving inflammation mitigation, epithelial barrier restoration, and retardation of colitis and its associated tumorigenesis. As an LNP-based oral RNP delivery system, TPGS-RNP@LNPs provide a promising platform for precise treatment of colorectal diseases.
Figures

References
-
- Gros B., Kaplan G. G., Ulcerative colitis in adults: A review. JAMA 330, 951–965 (2023). - PubMed
-
- Li X. V., Leonardi I., Putzel G. G., Semon A., Fiers W. D., Kusakabe T., Lin W. Y., Gao I. H., Doron I., Gutierrez-Guerrero A., DeCelie M. B., Carriche G. M., Mesko M., Yang C., Naglik J. R., Hube B., Scherl E. J., Iliev I. D., Immune regulation by fungal strain diversity in inflammatory bowel disease. Nature 603, 672–678 (2022). - PMC - PubMed
-
- Gillmore J. D., Gane E., Taubel J., Kao J., Fontana M., Maitland M. L., Seitzer J., O’Connell D., Walsh K. R., Wood K., Phillips J., Xu Y., Amaral A., Boyd A. P., Cehelsky J. E., McKee M. D., Schiermeier A., Harari O., Murphy A., Kyratsous C. A., Zambrowicz B., Soltys R., Gutstein D. E., Leonard J., Sepp-Lorenzino L., Lebwohl D., CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N. Engl. J. Med. 385, 493–502 (2021). - PubMed
-
- Liu Y., Zeng Y., Liu L., Zhuang C., Fu X., Huang W., Cai Z., Synthesizing and gate genetic circuits based on CRISPR-Cas9 for identification of bladder cancer cells. Nat. Commun. 5, 5393 (2014). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

