Correlates of risk of respiratory syncytial virus disease: a prospective cohort study
- PMID: 41006233
- PMCID: PMC12475016
- DOI: 10.1038/s41467-025-63434-x
Correlates of risk of respiratory syncytial virus disease: a prospective cohort study
Abstract
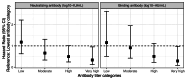
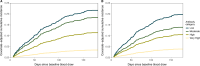
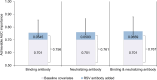
Few population-based studies have evaluated the importance of pre-existing respiratory syncytial virus (RSV) antibody on RSV susceptibility among children and adults. We conducted a prospective, community-based cohort study among individuals aged 6 months-50 years in Oregon and Washington State, USA (June 2022-May 2023), with weekly symptom surveys and swab collection regardless of symptoms. Swabs were tested for RSV using RT-qPCR. Enrollment sera were tested for RSV prefusion F IgG binding (all participants) and neutralizing antibodies (pediatric participants). We detected 305 RSV illnesses among 3237 participants from 1188 households. Using proportional hazards regression, higher RSV binding antibody titers were associated with a lower estimated hazard of RSV among pediatric participants (hazard ratio=0.66 per 1-unit difference in log10-RSV antibody titer; 95% CI: 0.56, 0.78). In a post-pandemic period, pre-existing RSV antibody titers were associated with a lower risk of RSV illness in children aged 6 months-17 years, which could inform vaccine development for this age group.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.N.C. reported consulting with Pfizer, Inc. outside of the submitted work. C.M.L. reported being on the Scientific Advisory Board for LGC group. J.L.K. reported research funding not related to the submitted work from Pfizer, Novartis, and Vir Biotechnology. R.A.M. reported research funding not related to the submitted work from PCORI, CDC, NHLBI, NIAID, Glaxo Smith Kline, Merck, and Sanofi; his institution is funded by Pfizer, Inc. for RSV vaccine development as site PI. A.L.G. reported central lab contract testing from Abbott, Cepheid, Novavax, Pfizer, Janssen and Hologic, research support from Gilead, outside of the described work. A.A.W. reported research funding unrelated to this work from Pfizer, Inc. J.A.E. reported consulting with Ark Biopharmaceuticals, Sanofi Pasteur, Moderna, Meissa Vaccines, Astra Zeneca, and Pfizer, Inc. outside of the submitted work, and has received research funding from AstraZeneca, Merck, GlaxoSmithKline, and Pfizer. H.Y.C. reported consulting with Ellume, Pfizer, and the Bill and Melinda Gates Foundation; she has served on advisory boards for Vir, Merck and Abbvie; she has conducted CME teaching with Medscape, Vindico, and Clinical Care Options; she has received research funding from Gates Ventures, and support and reagents from Ellume and Cepheid outside of the submitted work. The other authors have no conflicts of interest to disclose.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

