Hypoxic conditioning in Parkinson's disease: randomized controlled multiple N-of-1 trials
- PMID: 41006236
- PMCID: PMC12475152
- DOI: 10.1038/s41467-025-63324-2
Hypoxic conditioning in Parkinson's disease: randomized controlled multiple N-of-1 trials
Abstract
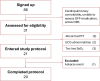
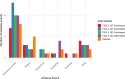
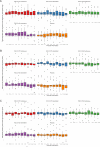
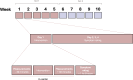
Preclinical evidence suggests positive symptomatic and neuroprotective effects of hypoxic conditioning in Parkinson's disease (PD). This study (NCT05214287) investigated the safety, feasibility, short-term symptomatic and downstream effects of hypoxic conditioning in individuals with PD. 20 individuals with PD (mean age 62, 10 women, Hoehn-Yahr 1.5-3) completed randomized controlled double-blinded multiple N-of-1 trials. Each participant underwent five different 45-minute hypoxia interventions in duplicate: continuous hypoxia at FiO2 0.163 and 0.127, intermittent (five-minute intervals interspersed with normoxia) at FiO2 0.163 and 0.127, and placebo. Primary outcomes were safety and feasibility as measured by adverse events, vital parameter disturbances, participant-rated discomfort and feasibility questionnaires. Secondary outcomes were short-term participant-rated and assessor-rated symptom scores. Exploratory indicators of target engagement were serum erythropoietin, brain-derived neurotrophic factor (BDNF), glial fibrillary acidic protein (GFAP), neurofilament light-chain (NfL), platelet-derived growth factor-receptor-β (PDGFRβ) and cortisol. Secondary outcomes were evaluated using frequentist and Bayesian analysis. 20 participants completed the protocol. The trial met its primary endpoints for safety and feasibility. 95 adverse events occurred, including one moderate and three serious events. Adverse events were not dose-dependent and occurred at comparable incidence following hypoxia and placebo. Hypoxic conditioning was well-tolerated. Low-FIO2 protocols caused significant oxygen desaturations in two participants. Participants considered longer-term application feasible. Intermittent hypoxia at FIO2 0.163 modestly improved most participant-rated symptoms for several hours compared to placebo, but not assessor-rated scales. One hour after intervention, serum markers did not differ between interventions. Hypoxic conditioning is safe and feasible in individuals with PD, and specific protocols may be associated with short-term symptom improvement. These findings inform and support follow-up studies of longer-term safety and efficacy of hypoxic conditioning.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Bastiaan R. Bloem has received honoraria from serving on the scientific advisory board for Abbvie, Biogen, UCB, and Walk with Path; has received fees for speaking at conferences from AbbVie, Zambon, Roche, GE Healthcare, and Bial; and has received research support from The Netherlands Organisation for Scientific Research, the Michael J. Fox Foundation, UCB, Abbvie, the Stichting Parkinson Fonds, the Hersenstichting Nederland, the Parkinson Foundation, Verily Life Sciences, Horizon 2020, the Topsector Life Sciences and Health, and the Parkinson Vereniging. The other authors have nothing to disclose.
Figures
References
-
- Broderick, P. A. & Gibson, G. E. Dopamine and serotonin in rat striatum during in vivo hypoxic-hypoxia. Metab. Brain Dis.4, 143–153 (1989). - PubMed
-
- Orset, C. et al. Dopamine transporters are involved in the onset of hypoxia-induced dopamine efflux in striatum as revealed by in vivo microdialysis. Neurochem. Int.46, 623–633 (2005). - PubMed
-
- Akiyama, Y. et al. Effects of hypoxia on the activity of the dopaminergic neuron system in the rat striatum as studied by in vivo brain microdialysis. J. Neurochem.57, 997–1002 (1991). - PubMed
-
- Witten, L. et al. HIF prolyl hydroxylase inhibition augments dopamine release in the rat brain in vivo. J. Neurosci. Res.87, 1686–1694 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

