Development of a novel assay for antigen presentation measurement
- PMID: 41006337
- PMCID: PMC12475488
- DOI: 10.1038/s41598-025-13997-y
Development of a novel assay for antigen presentation measurement
Abstract
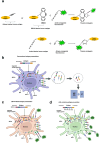
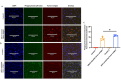
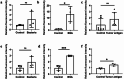
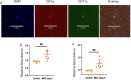
Accurate measurement of antigen presentation is essential for understanding immune responses to infections and tumors. However, current methods are cumbersome, time-consuming, and rely on known peptide sequences and antibodies, leading to unstable antigen presentation, antigen loss during processing and editing, and inconsistent results. We developed a novel, cost-effective method for examining antigen presentation using Click chemistry, which utilizes a bioorthogonal reaction between azides and alkynes/cyclooctenes. Antigens were pre-labeled with azides or alkynes to facilitate their uptake by antigen-presenting cells (APCs). Their presentation was subsequently detected using fluorophore-conjugated dibenzocyclooctyne or azide. The study involved three types of APCs, mouse macrophages (RAW264.7), mouse dendritic cells (DC2.4), and mouse primary bone marrow derived dendritic cells (BMDCs), and three categories of antigens: BSA, bacteria, and tumor antigens. Antigen presentation was measured and validated through multiple analytic techniques, including a fluorescent plate reader, flow cytometry, and ELISA. We showed efficient and stable presentation of antigens on the surface of all RAW264.7, DC2.4 and BMDCs. Antigens labeled using Click chemistry showed enhanced stability within the phagolysosomes of APCs. Notably, antigens labeled throughout the peptide sequence using azidohomoalanine (AHA) exhibited superior presentation on MHC class II compared to antigens labeled only at the N-terminus. Furthermore, this method preserved the natural antigen editing process, enabling the selection of high-affinity antigens for MHC presentation. This novel antigen presentation assay offers key advantages over existing methods, including faster processing, cost-effectiveness, stable antigen presentation, and reliable detection signals. When paired with mass spectrometry, it can identify stably presented tumor peptides, offering potential targets for immunotherapy development.
Keywords: Antigen presentation; Breast cancer; Click chemistry; MHC I/II; Phagocytosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

