Bimetallic [Co/K] hydrogen evolution catalyst for electrochemical terminal C-H functionalization
- PMID: 41006353
- PMCID: PMC12475264
- DOI: 10.1038/s41467-025-63914-0
Bimetallic [Co/K] hydrogen evolution catalyst for electrochemical terminal C-H functionalization
Abstract
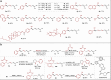
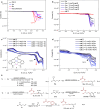
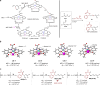
Discovering novel catalysts for hydrogen evolution reaction (HER) holds the potential to revolutionize the energy chemistry and unlock new tool for synthetic processes. Inspired by hydrogenases, we pair alkali metals with cobalt-Salen catalysts which allow the integration of naked base site into bimetallic HER catalysts. The incorporation of alkali metals (Na, K, Rb, Cs) significantly enhances HER activity. Among these, the [Co/K] system exhibits the highest HER catalytic efficiency (kobs ~ 31.4 s⁻¹), which is 9 times higher than the mononuclear analogue. Remarkably, this HER catalyst is repurposed for the terminal C(sp³)-H functionalization of N-allylimines with imine/aldehyde, a previously inaccessible transformation. Mechanistic studies reveal that the naked base site enables selective C-H activation via proton relay, overriding the inherent preference for Pinacol coupling. The electrochemical protocol features good functional group tolerance, and opens up a streamlined avenue for chiral pyrrolines, key precursors of the anti-cancer medicine Larotrectinib. More importantly, the alkali metal effect is rationalized through structural analysis, density functional theory (DFT) calculations, and control experiments.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Koper, M. T. M. & Bouwman, E. Electrochemical hydrogen production: bridging homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed.49, 3723–3725 (2010). - PubMed
-
- McKone, J. R., Marinescu, S. C., Brunchwig, B. S., Winkler, J. R. & Gray, H. B. Earth-abundant hydrogen evolution electrocatalysts. Chem. Sci.5, 865–878 (2014).
-
- Zhu, J., Hu, L., Zhao, P., Lee, L. Y. S. & Wong, K.-Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev.120, 851–918 (2020). - PubMed
-
- Zheng, Y., Jiao, Y., Vasileff, A. & Qiao, S.-Z. The hydrogen evolution reaction in alkaline solution: from theory, single crystal models, to practical electrocatalysts. Angew. Chem. Int. Ed.57, 7568–7579 (2018). - PubMed
-
- Tard, C. & Pickett, C. J. Structural and functional analogues of the active sites of the [Fe]-, [NiFe]-, and [FeFe]-hydrogenases. Chem. Rev.109, 2245–2274 (2009). - PubMed
Grants and funding
- 21702113/National Natural Science Foundation of China (National Science Foundation of China)
- 22422101, 22371002, 92061110/National Natural Science Foundation of China (National Science Foundation of China)
- S020318006/069/Anhui University
- 2308085Y14/Natural Science Foundation of Anhui Province (Anhui Provincial Natural Science Foundation)
LinkOut - more resources
Full Text Sources

