High Salt Intake Affects Visceral Adipose Tissue Homeostasis: Beneficial Effects of GLP-1 Agonists
- PMID: 41007316
- PMCID: PMC12467069
- DOI: 10.3390/biology14091171
High Salt Intake Affects Visceral Adipose Tissue Homeostasis: Beneficial Effects of GLP-1 Agonists
Abstract
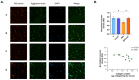
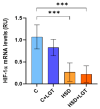
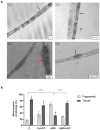
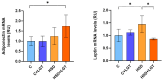
High salt (NaCl) intake has been associated with visceral adipose tissue (VAT) dysfunction independently of its impact on blood pressure. Liraglutide (LGT), a GLP-1 agonist, could be a potential therapeutic option. We investigated the impact of a chronic high-salt diet (HSD) on VAT homeostasis and evaluated the potential protective effects of LGT, a GLP-1 receptor agonist. Male C57BL/6 mice were fed a standard diet (Control, C) or 8% NaCl (HSD) for 15 weeks and subsequently treated with LGT or vehicle for 5 weeks. In VAT, histological characteristics, collagen deposition, vascular density, mitochondrial dynamics, oxidative stress, and adipokine expression were evaluated. The HSD significantly decreased body weight, VAT mass, and adipocyte size (p < 0.05). Moreover, it impaired vascular density and induced interstitial fibrosis (p < 0.01). LGT treatment improved vascularization and VEGF expression and reduced fibrosis (p < 0.05 vs. the HSD). The HSD induced oxidative stress and mitochondrial fragmentation, which were attenuated by LGT (p < 0.001). Leptin levels were elevated by the HSD (p < 0.05) and normalized with LGT, while adiponectin levels increased. In conclusion, excessive salt consumption induces structural and metabolic dysfunction in VAT. LGT therapy mitigates several of these adverse effects, supporting its potential as a novel strategy for managing salt-sensitive adipose tissue dysfunction.
Keywords: adipose tissue; fibrosis; high salt intake; liraglutide; vascular density.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Vollmer W., Sacks F.M., Ard J., Appel L.J., Bray G.A., Simons-Morton D.G., Conlin P.R., Svetkey L.P., Erlinger T.P., Moore T.J., et al. Effects of diet and sodium intake on blood pressure: Subgroup analysis of the DASH-sodium trial. Ann. Intern. Med. 2001;135:1019–1028. doi: 10.7326/0003-4819-135-12-200112180-00005. - DOI - PubMed
-
- World Health Organization . Guideline: Sodium Intake for Adults and Children. World Health Organization; Geneva, Switzerland: 2012. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

