Microglial Autophagy and Mitophagy in Ischemic Stroke: From Dual Roles to Therapeutic Modulation
- PMID: 41007413
- PMCID: PMC12467092
- DOI: 10.3390/biology14091269
Microglial Autophagy and Mitophagy in Ischemic Stroke: From Dual Roles to Therapeutic Modulation
Abstract
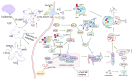
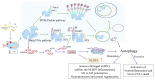
Ischemic stroke induces complex neuroinflammatory cascades, where microglial autophagy and mitophagy serve dual roles in both injury amplification and tissue repair. This scoping review synthesized current evidence on their regulatory mechanisms and therapeutic implications. Literature was identified via PubMed and Embase, yielding 79 records, from which 39 original research articles and 13 review papers were included after eligibility screening. Search terms included "microglia," "autophagy," and "ischemic stroke." Protective autophagy was frequently associated with AMPK activation, mTOR inhibition, and mitophagy pathways such as PINK1/Parkin and BNIP3/NIX, facilitating mitochondrial clearance, M2 polarization, and anti-inflammatory signaling. Therapeutic agents such as rapamycin, Tat-Beclin 1, and Urolithin A consistently demonstrated neuroprotection in preclinical stroke models. In contrast, excessive or prolonged autophagic activation was linked to inflammasome amplification, oxidative stress, and phagoptosis. Limited human studies reported associations between elevated serum ATG5 levels or ATG7 polymorphisms and worse clinical outcomes, suggesting preliminary translational relevance. These findings support the potential of phase-specific modulation of microglial autophagy as a therapeutic avenue for stroke, although further validation in human models and development of autophagy biomarkers are needed for clinical application.
Keywords: M1/M2 polarization; PINK1/Parkin; autophagy; inflammasome; ischemic stroke; microglia; mitophagy; neuroinflammation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Saceleanu V.M., Toader C., Ples H., Covache-Busuioc R.A., Costin H.P., Bratu B.G., Dumitrascu D.I., Bordeianu A., Corlatescu A.D., Ciurea A.V. Integrative Approaches in Acute Ischemic Stroke: From Symptom Recognition to Future Innovations. Biomedicines. 2023;11:2617. doi: 10.3390/biomedicines11102617. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

