Pharmaco-Epigenetics and Epigenetic Drugs in Type 2 Diabetes: Can Epigenetics Predict Drug Efficiency?
- PMID: 41007838
- PMCID: PMC12467409
- DOI: 10.3390/biomedicines13092278
Pharmaco-Epigenetics and Epigenetic Drugs in Type 2 Diabetes: Can Epigenetics Predict Drug Efficiency?
Abstract
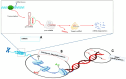
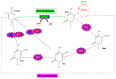
Type 2 Diabetes Mellitus (T2DM) is increasingly affecting individuals across various age groups due to inadequate insulin action and secretion. It has become the leading cause of mortality worldwide, with an estimated 9.3% of the global population currently affected. Recent epigenetic studies have shown that variations such as DNA methylation and histone modifications are implicated in the development of T2DM. However, epigenetically related conditions are known to be reversible, which could potentially pave the way for predicting and treating T2DM. This has led to the development of epigenetic modifier drugs, including histone deacetylase inhibitors (HDACi), histone acetyltransferase inhibitors (HATi), protein arginine methyltransferase inhibitors (PRMTi), DNA methyltransferase inhibitors (DNMTi), histone demethylating inhibitors (HDMi), and sirtuin-activating compounds (STAC). A major challenge with these epigenetic drugs is that only a few have been approved for treating metabolic diseases due to their potential to negatively impact off-target genes. The low specificity of these drugs can lead to side effects and increased toxicity, contributing to complex diseases such as cancer. Hence, gaining a comprehensive understanding of the epigenetic mechanisms underlying metabolic diseases can provide new insights and strategies for preventing, diagnosing, and treating metabolic disorders, such as T2DM. This review summarizes the epigenetic variations in T2DM, pharmaco-epigenetics, and the challenges surrounding epigenetics. This provides basic insight into the discovery of novel drug targets, which can lead to the development of epigenetic therapies for T2DM. Hence, the reversible nature of epigenetic variations retains hope for future novel strategies to combat T2DM.
Keywords: DNA methylation; diabetes mellitus; epi-drugs; epigenetics; histone modifications.
Conflict of interest statement
The authors declare no conflicts of interest for this manuscript.
Figures
References
-
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. - DOI - PubMed
-
- Sifunda S., Mbewu A.D., Mabaso M., Manyaapelo T., Sewpaul R., Morgan J.W., Harriman N.W., Williams D.R., Reddy S.P. Prevalence and Psychosocial Correlates of Diabetes Mellitus in South Africa: Results from the South African National Health and Nutrition Examination Survey (SANHANES-1) Int. J. Environ. Res. Public Health. 2023;20:5798. doi: 10.3390/ijerph20105798. - DOI - PMC - PubMed
-
- Tan S.Y., Mei Wong J.L., Sim Y.J., Wong S.S., Mohamed Elhassan S.A., Tan S.H., Ling Lim G.P., Rong Tay N.W., Annan N.C., Bhattamisra S.K., et al. Type 1 and 2 Diabetes Mellitus: A Review on Current Treatment Approach and Gene Therapy as Potential Intervention. Diabetes Metab. Syndr. Clin. Res. Rev. 2019;13:364–372. doi: 10.1016/j.dsx.2018.10.008. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

