CD45 and Basigin (CD147) Are Functional Ligands for Galectin-8 on Human Leukocytes
- PMID: 41008550
- PMCID: PMC12467718
- DOI: 10.3390/biom15091243
CD45 and Basigin (CD147) Are Functional Ligands for Galectin-8 on Human Leukocytes
Abstract
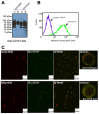
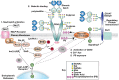
The interactions of leukocyte glycoproteins with adhesion and signaling molecules through glycan recognition are not well understood. We previously demonstrated that galectin-8, a tandem-repeat lectin with N- and C-terminal carbohydrate binding domains which is highly expressed in endothelial and epithelial cells, can bind to activated neutrophils to induce surface exposure of phosphatidylserine (PS) without DNA fragmentation or apoptosis, in a process termed preaparesis. However, the receptors for Gal-8 on leukocytes have not been identified. Here we report our results using both proteomics and affinity chromatography with both full-length Gal-8 and the separate Gal-8 C-terminal and N-terminal domains to identify glycoprotein ligands in HL-60 cells for Gal-8. Two of the major ligands for Gal-8 are CD45RA and CD45RC (Protein Tyrosine Phosphatase, PTP) and basigin (CD147). Both CD45 and basigin are integral membrane glycoproteins that carry poly-N-acetyllactosamine modifications on N- and/or O-glycans, required for Gal-8 binding. Inhibition of the phosphatase activity of CD45 reduced Gal-8-induced PS exposure, indicating a possible role of CD45 in Gal-8 signaling of preaparesis in human leukocytes. These results demonstrate unique glycoprotein recognition by Gal-8 involved in cell recognition and signaling.
Keywords: CD45; basigin; galectin-8; glycan ligands; leukocytes.
Conflict of interest statement
Author Alexander J. Noll was employed by The Emmes Company, LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Cummings R.D., Rabinovich G.A., Stowell S.R., Vasta G. Galectins. In: Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Mohnen D., Kinoshita T., Packer N.H., Prestegard J.H., et al., editors. Essentials of Glycobiology. 4th ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2022.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

