Approach to Studies on Podocyte Lesions Mediated by Hyperglycemia: A Systematic Review
- PMID: 41009556
- PMCID: PMC12469789
- DOI: 10.3390/ijms26188990
Approach to Studies on Podocyte Lesions Mediated by Hyperglycemia: A Systematic Review
Abstract
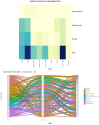
Podocyte injury is a central event in the pathogenesis of diabetic nephropathy (DN). We conducted a systematic review across four major databases, identifying 7769 records and including 130 studies that met predefined eligibility criteria. Methodological quality was assessed with Joanna Briggs Institute tools, yielding a mean score of 81.3%, indicating overall moderate-to-high rigor despite design-contingent limitations. Publication activity was sparse until 2018 but increased markedly thereafter, with more than 80% of studies published between 2019 and 2025. Temporal analyses confirmed a strong positive trend (p = 0.86, p < 0.0001), reflecting the rapid expansion of this field. Study designs evolved from early human-only descriptions to integrated multi-model approaches combining human tissue, animal experiments, and in vitro systems, thus balancing clinical relevance with mechanistic exploration. Geographically, Asia emerged as the leading contributor, complemented by increasing multinational collaborations. Mechanistic synthesis highlighted five reproducible pillars of podocyte injury: slit-diaphragm and adhesion failure, mTOR-autophagy-ER stress disequilibrium, mitochondrial and lipid-driven oxidative injury, immune, complement, and inflammasome activation, and epigenetic and transcriptomic reprogramming. Collectively, these findings underscore a convergent mechanistic cascade driving podocyte dysfunction, while also providing a framework for therapeutic interventions aimed at restoring barrier integrity, metabolic balance, and immune regulation in DN.
Keywords: diabetic neuropathy; physiopathology; podocytes.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Parchwani D.N., Upadhyah A.A. Diabetic nephropathy: Progression and pathophysiology. Int. J. Med. Sci. Public Health. 2012;1:59–70. doi: 10.5455/ijmsph.2012.1.59-70. - DOI
-
- Fried L.F., Folkerts K., Smela B., Deon Bowrin K., Mernagh P., Millier A., Kovesdy C.P. Targeted literature review of the burden of illness in patients with chronic kidney disease and type 2 diabetes. Am. J. Manag. Care. 2021;27:S168–S177. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

