Teashirt and C-Terminal Binding Protein Interact to Regulate Drosophila Eye Development
- PMID: 41009990
- PMCID: PMC12469452
- DOI: 10.3390/genes16091045
Teashirt and C-Terminal Binding Protein Interact to Regulate Drosophila Eye Development
Abstract
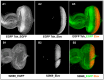
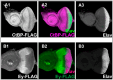
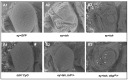
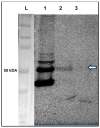
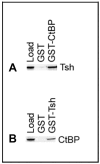
Background and Objectives: The Drosophila retinal determination network comprises the transcription factor Teashirt (Tsh) and the transcription co-regulator C-terminal Binding Protein (CtBP), both of which are essential for normal adult eye development. Both Tsh and CtBP show a pattern of co-expression in the proliferating cells anterior to the morphogenetic furrow that demarcates the boundary between the anteriorly placed proliferating eye precursor cells and the posteriorly placed differentiating photoreceptor cells in the larval eye-precursor tissue, the eye-antennal disc. The disc ultimately develops into the adult compound eyes, antenna, and other head structures. Both Tsh and CtBP were found to interact genetically during ectopic eye formation in Drosophila, and both were present in molecular complexes purified from gut and cultured cells. However, it remained unknown whether Tsh and CtBP molecules could interact in the eye-antennal discs and elicit an effect on eye development. The present study answers these questions. Methods: 5' GFP-tagging of the tsh gene in the Drosophila genome and 5' FLAG-tagging of the ctbp gene were accomplished by the CRISPR-Cas9 and BAC recombineering methods, respectively, to produce GFP-Tsh- and FLAG-CtBP-fused proteins in specific transgenic Drosophila strains. Verification of these proteins' expression in the larval eye-antennal discs was performed by immunohistological staining and confocal microscopy. Genetic screening was performed to establish functional interaction between Tsh and CtBP during eye development. Scanning Electron Microscopy was performed to image the adult eye structure. Co-immunoprecipitation and GST pulldown assays were performed to show that Tsh and CtBP interact in the cells of the third instar eye-antennal discs. Results: This study reveals that Tsh and CtBP interact genetically and physically in the Drosophila third instar larval eye-antennal disc to regulate adult eye development. This interaction is likely to limit the population of the eye precursor cells in the larval eye disc of Drosophila. Conclusions: The relative abundance of Tsh and CtBP in the third instar larval eye-antennal disc can dictate the outcome of their interaction on the Drosophila eye formation.
Keywords: BAC recombineering; C-terminal binding protein; CRISPR-Cas9; Drosophila; GST pulldown; co-immunoprecipitation; eye development; genetic interaction; teashirt.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Pappu K., Mardon G. Results and Problems in Cell Differentiation. Volume 37. Springer; Berlin/Heidelberg, Germany: 2002. Retinal specification and determination in Drosophila; pp. 5–20. - PubMed
-
- Fristrom D., Fristrom J.W. The Metamorphic Development of the Adult Epidermis. Volume 1–2 CSH Press; New York, NY, USA: 1993.
MeSH terms
Substances
Grants and funding
- P20 GM103429/GM/NIGMS NIH HHS/United States
- T34 GM007667/GM/NIGMS NIH HHS/United States
- NIH award no. GM07667-41, Arkansas INBRE program, with a grant from the National Institute of General Medical Sciences (NIGMS), P20 GM103429 from the NIH, and Texas Tech University New Faculty Start-up Funds./GF/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

