De Novo Transcriptome Sequencing and Profiling of Ovarian Development of Argas persicus Along the Trophogonic Cycle
- PMID: 41010052
- PMCID: PMC12469708
- DOI: 10.3390/genes16091107
De Novo Transcriptome Sequencing and Profiling of Ovarian Development of Argas persicus Along the Trophogonic Cycle
Abstract
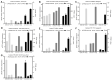
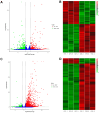
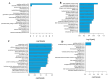
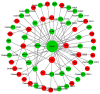
Background: Argas persicus is a hematophagous ectoparasite of poultry and is the vector of several agents infectious to poultry. This study aims to explore the key genes affecting the ovarian development of A. persicus. Methods: RNA-seq was performed on the ovaries of A. persicus before blood-feeding, on the day of engorgement, and 6 days post-engorgement. Utilizing the threshold padj < 0.05 and|log2(foldchange)| > 1, differentially expressed genes were identified, and hub genes were determined by constructing protein-protein interaction (PPI) networks. Results: A total of 1008 differentially expressed genes were obtained during the feeding period, including 448 up-regulated and 560 down-regulated genes. Further, 2179 differentially expressed genes were screened in the preoviposition stage, including 1957 up-regulated and 222 down-regulated genes. These genes are mainly annotated in functions such as peptidase activity (especially serine protease activity), protein folding, protein assembly, and cell component assembly, and enriched in pathways such as protein processing in endoplasmic reticulum, lysosome, glutathione metabolism, and sphingolipid metabolism. In addition, some proteins that are closely related to ovarian development, including heat shock protein 70, protein disulfide isomerase, paramyosin, troponin I, hexosaminidase, serine protease, Kunitz serine protease inhibitors, and vitellogenin, were obtained. Conclusions: These findings fill the gap in the biological data for the ovarian development of soft ticks, provide a reference database for subsequent proteomics research, and offer fundamental support for the screening and development of candidate antigens for anti-tick vaccines.
Keywords: Argas persicus; differentially expressed genes; ovary; transcriptome.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures

References
-
- Kohls G.M., Hoogstraal H., Clifford C.M., Kaiser M.N. The Subgenus Persicargas (Ixodoidea, Argasidae, Argas). 9. Redescription and New World Records of Argas (P.) persicus (Oken), and Resurrection, Redescription, and Records of A. (P.) radiatus Railliet, A. (P.) sanchezi Duges, and A. (P.) miniatus Koch, New World Ticks Misidentified as A. (P.) persicus. Ann. Entomol. Soc. Am. 1970;63:590–606. doi: 10.1093/aesa/63.2.590. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

