Dual-Action Grouper Bone and Wakame Hydrolysates Supplement Enhances Exercise Performance and Modulates Gut Microbiota in Mice
- PMID: 41010459
- PMCID: PMC12472609
- DOI: 10.3390/nu17182933
Dual-Action Grouper Bone and Wakame Hydrolysates Supplement Enhances Exercise Performance and Modulates Gut Microbiota in Mice
Abstract
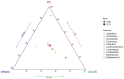
Background: Sustainable, dual-action ergogenic strategies are underexplored; most products target a single pathway and rarely upcycle seafood sidestreams. We therefore tested an upcycled formulation combining grouper bone hydrolysate and Undaria pinnatifida extract (GU) for ergogenic and microbiota effects in mice. We tested the ergogenic and microbiota modulating effects of GU in mice versus a vehicle and a BCAA control. Methods: GU was prepared via enzymatic hydrolysis of marine by-products and administered to male ICR mice for 4 weeks. Mice were divided into five groups (n = 7/group), receiving a vehicle control, a branched-chain amino acid (BCAA) supplement, or GU at three dose levels (1X, 2X, 3X) based on human-equivalent conversion. Exercise performance was assessed via grip strength and treadmill tests. Biochemical markers of fatigue, body composition, and safety indicators were also analyzed. Gut microbiota was evaluated using 16S rRNA sequencing and constrained principal coordinates analysis (CPCoA). Results: Four weeks of GU supplementation significantly enhanced exercise performance [(treadmill time ↑ Δ = 10.2-11.7 min versus vehicle (q ≤ 0.0002), grip strength ↑ Δ = 40.4-48.5 g (q ≤ 0.05)] and lean body mass [FFM ↑ at GU-1X (Δ = +0.80%, q = 0.0123)], surpassing the commercial BCAA control. Biochemical analyses indicated reduced exercise-induced lactate accumulation [(post-exercise lactate ↓ Δ = -2.71/-2.18 mmol·L-1, q = 0.0006)]. Gut microbiota profiling revealed distinct shifts in community composition in GU-treated groups, notably with an increased abundance of beneficial taxa such as Lactobacillus and Muribaculum. These alterations reflect the prebiotic activity of seaweed-derived polysaccharides, promoting a healthier gut microbial profile. Notably, GU improved metabolic markers (aspartate aminotransferase, [AST]; lactate dehydrogenase, [LDH]) without inducing toxicity. Conclusions: These findings indicate that GU functions as a dual-action supplement, coupling amino acid-mediated muscle anabolism with microbiome modulation to enhance physical performance and metabolic health. As an upcycled marine product, it presents a sustainable and effective strategy for exercise support. Future studies should include 90-day safety, mechanistic assays, and a preregistered human pilot.
Keywords: Undaria pinnatifida; exercise performance; grouper bone; gut microbiota; microbiome modulation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Wimmer B.C., Dwan C., De Medts J., Duysburgh C., Rotsaert C., Marzorati M. Undaria pinnatifida fucoidan enhances gut microbiome, butyrate production, and exerts anti-inflammatory effects in an in vitro short-term shime(®) coupled to a caco-2/thp-1 co-culture model. Mar. Drugs. 2025;23:242. doi: 10.3390/md23060242. - DOI - PMC - PubMed
-
- Bangar S.P., Chaudhary V., Kajla P., Balakrishnan G., Phimolsiripol Y. Strategies for upcycling food waste in the food production and supply chain. Trends Food Sci. Technol. 2024;143:104314
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

