Carrot Rhamnogalacturonan-I Supplementation Shapes Gut Microbiota and Immune Responses: A Randomised Trial in Healthy Adults
- PMID: 41011488
- PMCID: PMC12472267
- DOI: 10.3390/microorganisms13092156
Carrot Rhamnogalacturonan-I Supplementation Shapes Gut Microbiota and Immune Responses: A Randomised Trial in Healthy Adults
Abstract
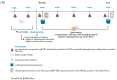
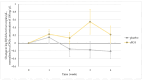
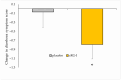
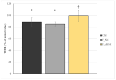
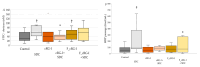
Background: Rhamnogalacturonan-I (RG-I) is a pectic polysaccharide with emerging prebiotic and immunomodulatory potential. This randomised, double-blind, placebo-controlled trial (ID: NCT06081972) evaluated the effects of carrot-derived RG-I (cRG-I) supplementation, compared to placebo (maltodextrin), on gut microbiota composition and immune cell activation in healthy adults. Methods: A total of 54 participants (18-70 years old) were randomised in a double-blind manner to receive either 500 mg/day of cRG-I or placebo for four weeks. Pre-screening ensured balanced randomisation based on habitual fibre intake and faecal Bifidobacterium counts. Questionnaires assessed potential gut health and well-being effects, while in vitro and ex vivo models were used to evaluate effects on intestinal permeability. Results: cRG-I was well tolerated with excellent compliance. Faecal Bifidobacterium counts increased significantly, peaking at week 3. Isobutyric acid levels rose, though no other SCFAs differed. Immunologically, cRG-I enhanced the percentage of circulating myeloid dendritic cells expressing activation markers (CD86, HLA-DR) on. Stool consistency improved slightly. Preclinical models further showed that cRG-I and its fermentation products protected intestinal barrier integrity under stress. Conclusion: These results support cRG-I as a safe, low-dose dietary intervention capable of beneficially modulating gut microbiota, immune responses, and barrier function in healthy adults within a short supplementation period.
Keywords: DC activation; bifidobacteria; cRG-I; diarrhoea score; healthy adults; prebiotic; rhamnogalacturonan-I.
Conflict of interest statement
The authors declare no conflicts of interest. NutriLeads provided the cRG-I material used in this study. S.M. is an employee of NutriLeads. A.M. and R.A. are co-founders and shareholders of NutriLeads.
Figures


References
-
- World Health Organization . Carbohydrate Intake for Adults and Children: WHO Guideline Summary. World Health Organization; Geneva, Switzerland: 2023. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

