Hans Paulsen: Contributions to the Investigations of Glycoprotein Biosynthesis
- PMID: 41011627
- PMCID: PMC12472385
- DOI: 10.3390/molecules30183735
Hans Paulsen: Contributions to the Investigations of Glycoprotein Biosynthesis
Abstract
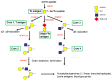
Hans Paulsen was one of the first scientists who believed that chemistry should be applied to biology and medicine. His interest in natural products and their roles solidified in the 1970s. He passed on his knowledge to hundreds of students and coworkers and advanced science with many national and international collaborators. No matter where he was, at home or travelling, he was always curious and keen to learn, from chemistry to enzymes, their roles in diseases, and the possible applications of synthetic compounds. His creative chemistry and synthesis of novel compounds made essential contributions to elucidating the mechanisms and pathways of glycoprotein biosynthesis. This review describes the biosynthetic pathways of the O- and N-glycans of glycoproteins and studies of novel substrates and inhibitors developed by Hans Paulsen's group.
Keywords: N-glycosylation; O-glycosylation; glycopeptides; glycoproteins; glycosyltransferases; inhibitors; mucins; substrates.
Conflict of interest statement
The author declares no conflicts of interest.
Figures




References
-
- Heyns K., Paulsen H. Oxidative transformation of carbohydrates. VIII. Catalytic oxidation of meso-inositol to scyllo-meso-inosose. Chem. Berichte Chem. Eur. 1953;86:833–840. doi: 10.1002/cber.19530860702. - DOI
-
- Heyns K., Paulsen H., Rudiger G., Weyer J. Configuration and conformation selectivity in catalytic oxidation with oxygen on platinum catalysts. Fortschritte Der Chem. Forsch. 1969;11:285–374.
-
- Paulsen H., Todt K. Monosaccharides with a nitrogen-containing ring. XV. Nuclear magnetic resonance spectroscopic studies of hindered rotation of monosaccharides with a nitrogen-containing ring. Chem. Berichte Chem. Eur. 1967;100:3397–3404. doi: 10.1002/cber.19671001030. - DOI
-
- Paulsen H., Hayauchi Y., Sinnwell V. Monosaccharides containing nitrogen in the ring. XXXVII. Synthesis of 1,5-dideoxy-1,5-imino-D-galactitol. Chem. Berichte Chem. Eur. 1980;113:2601–2608. doi: 10.1002/cber.19801130803. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

