Thiazolylcyanocyclopropanes: Novel Donor-Acceptor Cyclopropanes for Accessing Thiazole-Containing Targets
- PMID: 41011659
- PMCID: PMC12472231
- DOI: 10.3390/molecules30183767
Thiazolylcyanocyclopropanes: Novel Donor-Acceptor Cyclopropanes for Accessing Thiazole-Containing Targets
Abstract
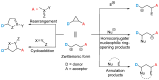
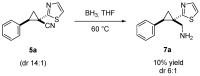
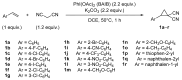
Donor-acceptor (D-A) cyclopropanes are important precursors in the synthesis of complex molecules due to their bidentate character and high reactivity. Among them, cyclopropane-1,1-dicarbonitriles are less commonly reported in modern literature, primarily because of the high reactivity of the nitrile groups and their limited compatibility with metal-catalyzed processes, which is caused by the geometrical constraints imposed by the linear cyano substituents. While the cyano groups can be seen as a limitation, they also offer synthetic versatility by serving as handles for further functionalization. In this work, we performed a cycloaddition reaction with mercaptoacetaldehyde, leading to a new class of DA cyclopropanes bearing a thiazole moiety. This one-pot, two-step transformation requires only a single purification step. The resulting thiazolyl-substituted cyclopropanes were subjected to ring strain-release reactions, showing reactivity comparable to the parent cyclopropane-1,1-dicarbonitriles.
Keywords: cycloaddition; donor–acceptor cyclopropanes; phase-transfer catalysis; ring-strain release; thiazoles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- de Meijere A. Bonding properties of cyclopropane and their chemical consequences. Angew. Chem. Int. Ed. Engl. 1979;18:809–826. doi: 10.1002/anie.197908093. - DOI
-
- Tomilov Y.V., Menchikov L.G., Novikov R.A., Ivanova O.A., Trushkov I.V. Methods for the synthesis of donor-acceptor cyclopropanes. Russ. Chem. Rev. 2018;87:201–250. doi: 10.1070/RCR4787. - DOI
-
- Banerjee P., Biju A.T. Donor-Acceptor Cyclopropanes in Organic Synthesis. 1st ed. Wiley-VCH GmbH; Weinheim, Germany: 2024.
LinkOut - more resources
Full Text Sources

