Cross-Neutralization of Distant Coronaviruses Strongly Correlates with Spike S2-Specific Antibodies from Immunocompetent and Immunocompromised Vaccinated SARS-CoV-2-Infected Patients
- PMID: 41012152
- PMCID: PMC12474146
- DOI: 10.3390/vaccines13090949
Cross-Neutralization of Distant Coronaviruses Strongly Correlates with Spike S2-Specific Antibodies from Immunocompetent and Immunocompromised Vaccinated SARS-CoV-2-Infected Patients
Abstract
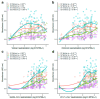
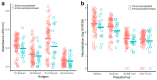
Background/Objectives: Despite the lifting of the COVID-19 public health emergency, SARS-CoV-2 infections continue to be recorded worldwide. The continued prevalence of infection has been attributed to the ability of the virus to evade host immune responses, including neutralizing antibody-derived immunity. The vast majority of antibody escape mutations has been associated with the S1 subunit of the spike protein. The other region of the spike, the S2 subunit, is the most conserved region amongst coronaviruses. We hypothesized that S2-specific antibody levels are modest in vaccinated and SARS-CoV-2-infected patients, resulting in suboptimal neutralization of distant coronaviruses. Methods: Here, we analyzed S1- and S2-specific antibody levels in SARS-CoV-2-infected individuals, including a mixed cohort of those with and without immunosuppression and prior vaccination. Results: We found that S2-specific antibody responses were generally lower than S1-specific antibody responses. Intriguingly, Omicron-S1-specific antibody levels were higher than Wuhan-S1-specific antibody levels despite all vaccinated participants having received Wuhan-spike-based immunogens. This emphasizes the importance of the infecting variant and vaccine immunogen in the production of spike-targeting antibodies and associated hybrid immunity. Although S1-specific antibody levels were generally higher than their S2-specific counterparts, the correlation between neutralization and binding antibody levels was mostly higher in S2- compared with S1-specific responses. Conclusions: We conclude that S2-based immunogens are suitable for the induction of antibody-based immunity against novel SARS-CoV-2 variants but also against more distant coronaviruses, which would support a better protection for the immunocompromised as well as other vulnerable populations.
Keywords: COVID-19; S2-specific antibodies; SARS-CoV-2; immunocompromised; neutralizing antibodies; spike-S1 dominance.
Conflict of interest statement
The authors declare no competing interests. J.Z.L. has consulted for Abbvie and received a grant from Merck, but none of these activities impacted or influenced the design, performance, and conclusions of this study. J.A.S. has received research support from Boehringer Ingelheim and Bristol Myers Squibb unrelated to this work. He has performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer, ReCor, Sobi, and UCB unrelated to this work.
Figures



Update of
-
Cross-neutralization of distant coronaviruses correlates with Spike S2-specific antibodies from immunocompetent and immunocompromised vaccinated SARS-CoV-2-infected patients.Res Sq [Preprint]. 2024 Dec 5:rs.3.rs-5487774. doi: 10.21203/rs.3.rs-5487774/v1. Res Sq. 2024. Update in: Vaccines (Basel). 2025 Sep 04;13(9):949. doi: 10.3390/vaccines13090949. PMID: 39678346 Free PMC article. Updated. Preprint.
References
-
- Stawicki S.P., Jeanmonod R., Miller A.C., Paladino L., Gaieski D.F., Yaffee A.Q., De Wulf A., Grover J., Papadimos T.J., Bloem C., et al. The 2019-2020 Novel Coronavirus (Severe Acute Respiratory Syndrome Coronavirus 2) Pandemic: A Joint American College of Academic International Medicine-World Academic Council of Emergency Medicine Multidisciplinary COVID-19 Working Group Consensus Paper. J. Glob. Infect. Dis. 2020;12:47–93. doi: 10.4103/jgid.jgid_86_20. - DOI - PMC - PubMed
-
- Silk B.J., Scobie H.M., Duck W.M., Palmer T., Ahmad F.B., Binder A.M., Cisewski J.A., Kroop S., Soetebier K., Park M., et al. COVID-19 Surveillance After Expiration of the Public Health Emergency Declaration—United States, May 11, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023;72:523–528. doi: 10.15585/mmwr.mm7219e1. - DOI - PMC - PubMed
Grants and funding
- R01 AR077607/AR/NIAMS NIH HHS/United States
- P30 AR070253/GF/NIH HHS/United States
- P30 AR072577/AR/NIAMS NIH HHS/United States
- R01 AR080659/AR/NIAMS NIH HHS/United States
- U19 AI110818/GF/NIH HHS/United States
- R01 AR080659/GF/NIH HHS/United States
- SARS-CoV-2 Variants Program/Massachusetts Consortium on Pathogen Readiness
- P30 AR070253/AR/NIAMS NIH HHS/United States
- R01 AR077607/GF/NIH HHS/United States
- R01 AI176287/AI/NIAID NIH HHS/United States
- R01 AI176287/GF/NIH HHS/United States
- Ignite/Boston College Fund
- U19 AI110818/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

